
Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term does not refer to the single type of polymer but a group of polymers. Unlike polyethylene and polystyrene polyurethanes can be produced from a wide range of starting materials resulting various polymers within the same group. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

Polyether ether ketone (PEEK) is a colourless organic thermoplastic polymer in the polyaryletherketone (PAEK) family, used in engineering applications. It was invented in November 1978 and brought to market in the early 1980s by part of Imperial Chemical Industries (ICI) that later became Victrex PLC.

Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.
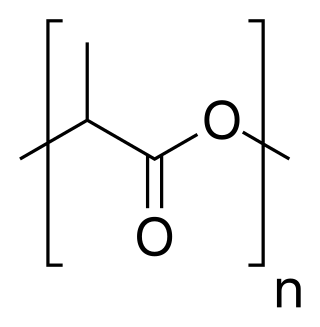
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a plastic material. As a thermoplastic polyester it has the backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n. PLA is formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit. Often PLA is blended with other polymers. PLA can be biodegradable or long-lasting, depending on the manufacturing process, additives and copolymers.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.
Polysulfones are a family of high performance thermoplastics. These polymers are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl-SO2-aryl subunit. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.

Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
In polymers, such as plastics, thermal degradation refers to a type of polymer degradation where damaging chemical changes take place at elevated temperatures, without the simultaneous involvement of other compounds such as oxygen. Simply put, even in the absence of air, polymers will begin to degrade if heated high enough. It is distinct from thermal-oxidation, which can usually take place at less elevated temperatures.
Polymer stabilizers are chemical additives which may be added to polymeric materials to inhibit or retard their degradation. Mainly they protect plastic and rubber products against heat, oxidation, and UV light. The biggest quantity of stabilizers is used for polyvinyl chloride (PVC), as the production and processing of this type of plastic would not be possible without stablizing chemicals. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.

In polymer chemistry photo-oxidation is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break, resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.
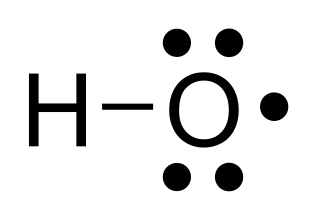
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
In polymer chemistry, in situ polymerization is a preparation method that occurs "in the polymerization mixture" and is used to develop polymer nanocomposites from nanoparticles. There are numerous unstable oligomers (molecules) which must be synthesized in situ for use in various processes. The in situ polymerization process consists of an initiation step followed by a series of polymerization steps, which results in the formation of a hybrid between polymer molecules and nanoparticles. Nanoparticles are initially spread out in a liquid monomer or a precursor of relatively low molecular weight. Upon the formation of a homogeneous mixture, initiation of the polymerization reaction is carried out by addition of an adequate initiator, which is exposed to a source of heat, radiation, etc. After the polymerization mechanism is completed, a nanocomposite is produced, which consists of polymer molecules bound to nanoparticles.
Biodegradable additives are additives that enhance the biodegradation of polymers by allowing microorganisms to utilize the carbon within the polymer chain as a source of energy. Biodegradable additives attract microorganisms to the polymer through quorum sensing after biofilm creation on the plastic product. Additives are generally in masterbatch formation that use carrier resins such as polyethylene (PE), polypropylene (PP), polystyrene (PS) or polyethylene terephthalate (PET).
Poly(ethylene adipate) or PEA is an aliphatic polyester. It is most commonly synthesized from a polycondensation reaction between ethylene glycol and adipic acid. PEA has been studied as it is biodegradable through a variety of mechanisms and also fairly inexpensive compared to other polymers. Its lower molecular weight compared to many polymers aids in its biodegradability.

High-performance plastics are plastics that meet higher requirements than standard (commodity) or engineering plastics. They are more expensive and used in smaller amounts.
Polyorthoesters are polymers with the general structure –[–R–O–C(R1, OR2)–O–R3–]n– whereas the residue R2 can also be part of a heterocyclic ring with the residue R. Polyorthoesters are formed by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane.
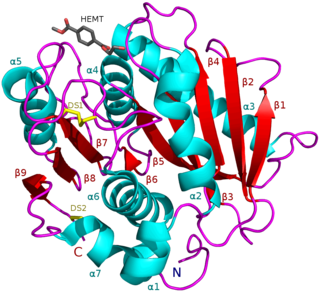
PETases are an esterase class of enzymes that catalyze the breakdown (via hydrolysis) of polyethylene terephthalate (PET) plastic to monomeric mono-2-hydroxyethyl terephthalate (MHET). The idealized chemical reaction is:
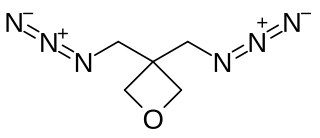
3,3-Bis(azidomethyl)oxetane (BAMO) is a oxetane monomer used in energetic propellant binders and plasticizer. It is frequently used as a copolymer to improve the physical properties of more commonly used polymers and to give them energetic properties.

Poly(trimethylene carbonate) (PTMC) is an aliphatic polycarbonate synthesized from the 6-membered cyclic carbonate, trimethylene carbonate (1,3-propylene carbonate or 1,3-Dioxan-2-one). Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255°C (at 760 mmHg). TMC is originally synthesized from 1,3-propanediol with phosgene or carbon monoxide, which are highly poisonous gases. Another route is from the transesterification of 1,3-propanediol and dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide.
Poly(phthalaldehyde), abbreviated as PPA, is a metastable stimuli-responsive polymer first synthesized in 1967. It has garnered significant attention during the past couple of years due to its ease of synthesis and outstanding transient and mechanical properties. for this reason, It has been exploited for a variety of applications including sensing, drug delivery, and EUV lithography. As of 2023, it is considered the only aromatic aldehyde polymerized through a living chain growth polymerization.












