Related Research Articles

In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Generally, fluorescent tagging, or labeling, uses a reactive derivative of a fluorescent molecule known as a fluorophore. The fluorophore selectively binds to a specific region or functional group on the target molecule and can be attached chemically or biologically. Various labeling techniques such as enzymatic labeling, protein labeling, and genetic labeling are widely utilized. Ethidium bromide, fluorescein and green fluorescent protein are common tags. The most commonly labelled molecules are antibodies, proteins, amino acids and peptides which are then used as specific probes for detection of a particular target.
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector. The sensitive biological element, e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc., is a biologically derived material or biomimetic component that interacts with, binds with, or recognizes the analyte under study. The biologically sensitive elements can also be created by biological engineering. The transducer or the detector element, which transforms one signal into another one, works in a physicochemical way: optical, piezoelectric, electrochemical, electrochemiluminescence etc., resulting from the interaction of the analyte with the biological element, to easily measure and quantify. The biosensor reader device connects with the associated electronics or signal processors that are primarily responsible for the display of the results in a user-friendly way. This sometimes accounts for the most expensive part of the sensor device, however it is possible to generate a user friendly display that includes transducer and sensitive element. The readers are usually custom-designed and manufactured to suit the different working principles of biosensors.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.
Nanosensors are nanoscale devices that measure physical quantities and convert these to signals that can be detected and analyzed. There are several ways proposed today to make nanosensors; these include top-down lithography, bottom-up assembly, and molecular self-assembly. There are different types of nanosensors in the market and in development for various applications, most notably in defense, environmental, and healthcare industries. These sensors share the same basic workflow: a selective binding of an analyte, signal generation from the interaction of the nanosensor with the bio-element, and processing of the signal into useful metrics.

Malachite green is an organic compound that is used as a dyestuff and controversially as an antimicrobial in aquaculture. Malachite green is traditionally used as a dye for materials such as silk, leather, and paper. Despite its name the dye is not prepared from the mineral malachite; the name just comes from the similarity of color.

A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microscope" refers to any microscope that uses fluorescence to generate an image, whether it is a simple set up like an epifluorescence microscope or a more complicated design such as a confocal microscope, which uses optical sectioning to get better resolution of the fluorescence image.
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol-gel process is used to produce ceramic nanoparticles.

Surface plasmon resonance (SPR) is the resonant oscillation of conduction electrons at the interface between negative and positive permittivity material in a particle stimulated by incident light. SPR is the basis of many standard tools for measuring adsorption of material onto planar metal surfaces or onto the surface of metal nanoparticles. It is the fundamental principle behind many color-based biosensor applications and lab-on-a-chip sensors. It should be stressed that SPR is not a resonance on the planar surface and it is a polariton or surface-wave like phenomenon.

In chemistry, solvatochromism is the phenomenon observed when the colour due to a solute is different when that solute is dissolved in different solvents.
Explosives trace detectors (ETD) are explosive detection equipment able to detect explosives of small magnitude. The detection is accomplished by sampling non-visible "trace" amounts of particulates. Devices similar to ETDs are also used to detect narcotics. The equipment is used mainly in airports and other vulnerable areas considered susceptible to acts of unlawful interference.

A molecular sensor or chemosensor is a molecular structure that is used for sensing of an analyte to produce a detectable change or a signal. The action of a chemosensor, relies on an interaction occurring at the molecular level, usually involves the continuous monitoring of the activity of a chemical species in a given matrix such as solution, air, blood, tissue, waste effluents, drinking water, etc. The application of chemosensors is referred to as chemosensing, which is a form of molecular recognition. All chemosensors are designed to contain a signalling moiety and a recognition moiety, that is connected either directly to each other or through a some kind of connector or a spacer. The signalling is often optically based electromagnetic radiation, giving rise to changes in either the ultraviolet and visible absorption or the emission properties of the sensors. Chemosensors may also be electrochemically based. Small molecule sensors are related to chemosensors. These are traditionally, however, considered as being structurally simple molecules and reflect the need to form chelating molecules for complexing ions in analytical chemistry. Chemosensors are synthetic analogues of biosensors, the difference being that biosensors incorporate biological receptors such as antibodies, aptamers or large biopolymers.

Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.
Fluorescent glucose biosensors are devices that measure the concentration of glucose in diabetic patients by means of sensitive protein that relays the concentration by means of fluorescence, an alternative to amperometric sension of glucose. Due to the prevalence of diabetes, it is the prime drive in the construction of fluorescent biosensors. A recent development has been approved by the FDA allowing a new continuous glucose monitoring system called EverSense, which is a 90-day glucose monitor using fluorescent biosensors.
Mesoporous organosilica are a type of silica containing organic groups that give rise to mesoporosity. They exhibit pore size ranging from 2 nm - 50 nm, depending on the organic substituents. In contrast, zeolites exhibit pore sizes less than a nanometer. PMOs have potential applications as catalysts, adsorbents, trapping agents, drug delivery agents, stationary phases in chromatography and chemical sensors.
An electro-switchable biosurface is a biosensor that is based on an electrode to which a layer of biomolecules has been tethered. An alternating or fixed electrical potential is applied to the electrode which causes changes in the structure and position (movement) of the charged biomolecules. The biosensor is used in science, e.g. biomedical and biophysical research or drug discovery, to assess interactions between biomolecules and binding kinetics as well as changes in size or conformation of biomolecules.
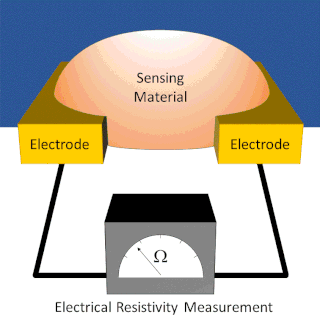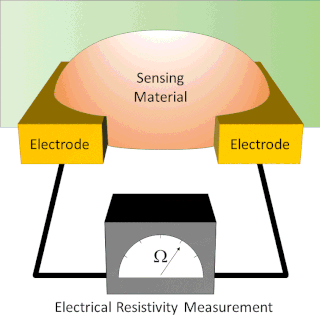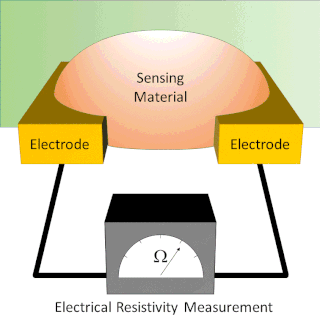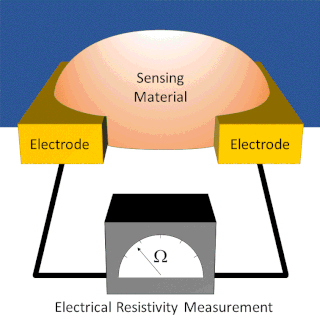
A chemiresistor is a material that changes its electrical resistance in response to changes in the nearby chemical environment. Chemiresistors are a class of chemical sensors that rely on the direct chemical interaction between the sensing material and the analyte. The sensing material and the analyte can interact by covalent bonding, hydrogen bonding, or molecular recognition. Several different materials have chemiresistor properties: metal-oxide semiconductors, some conductive polymers, and nanomaterials like graphene, carbon nanotubes and nanoparticles. Typically these materials are used as partially selective sensors in devices like electronic tongues or electronic noses.
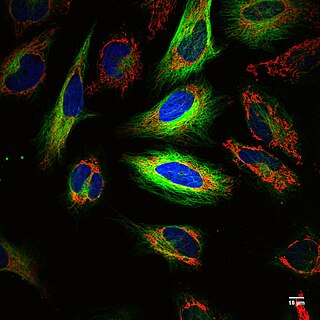
Fluorescence imaging is a type of non-invasive imaging technique that can help visualize biological processes taking place in a living organism. Images can be produced from a variety of methods including: microscopy, imaging probes, and spectroscopy.
A chemical sensor array is a sensor architecture with multiple sensor components that create a pattern for analyte detection from the additive responses of individual sensor components. There exist several types of chemical sensor arrays including electronic, optical, acoustic wave, and potentiometric devices. These chemical sensor arrays can employ multiple sensor types that are cross-reactive or tuned to sense specific analytes.
Photonic crystal sensors use photonic crystals: nanostructures composed of periodic arrangements of dielectric materials that interact with light depending on their particular structure, reflecting lights of specific wavelengths at specific angles. Any change in the periodicity or refractive index of the structure can give rise to a change in the reflected color, or the color perceived by the observer or a spectrometer. That simple principle makes them useful colorimetric intuitive sensors for different applications including, but not limited to, environmental analysis, temperature sensing, magnetic sensing, biosensing, diagnostics, food quality control, security, and mechanical sensing. Many animals in nature such as fish or beetles employ responsive photonic crystals for camouflage, signaling or to bait their prey. The variety of materials utilizable in such structures ranging from inorganic, organic as well as plasmonic metal nanoparticles makes these structures highly customizable and versatile. In the case of inorganic materials, variation of the refractive index is the most commonly exploited effect in sensing, while periodicity change is more commonly exhibited in polymer-based sensors. Besides their small size, current developments in manufacturing technologies have made them easy and cheap to fabricate on a larger scale, making them mass-producible and practical.
References
- ↑ "Sol-Gel Methods" (PDF).
- 1 2 Carrington, N. (2006). "Inorganic Sensing using Organofunctional Sol-Gel Materials". Acc. Chem. Res. 40 (5): 343–350. doi:10.1021/ar600017w. PMC 2041924 . PMID 17465520.
- ↑ Lopez, T. (1996). "Synthesis and Characterization of Sol-Gel Hydrotalcites Structure and Texture". Langmuir. 12: 189–192. doi:10.1021/la940703s.
- ↑ Prince, J. (2009). "Proposed General Sol-Gel Method to Prepare Multimetallic Layered Double Hydroxides: Synthesis, Characterization, and Envisaged Application". Chem. Mater. 21: 5826–5835. doi:10.1021/cm902741c.
- 1 2 Garcia-Heras, M. (2005). "Evaluation of Air Acidity through Optical Sensors". Environ. Sci. Technol. 39 (10): 3743–3747. Bibcode:2005EnST...39.3743G. doi:10.1021/es049558n. PMID 15952380.
- 1 2 Aernecke, M. (2009). "Design, Implementation, and Field Testing of a Portable Fluorescence-Based Vapor Sensor". Anal. Chem. 81 (13): 5281–5290. doi:10.1021/ac900505p. PMID 19563211.
- ↑ Hilderbrand, S. (2004). "Dirhodium Tetracarboxylate Scaffolds as Reversible Fluorescence-Based Nitric Oxide Sensors". J. Am. Chem. Soc. 126 (15): 4972–4978. doi:10.1021/ja038471j. PMID 15080703.
- ↑ Snyder, E. (2013). "The Changing Paradigm of Air Pollution Monitoring". Environ. Sci. Technol. 47 (20): 11369–11377. Bibcode:2013EnST...4711369S. doi:10.1021/es4022602. PMID 23980922.