
Arthritis is a term often used to mean any disorder that affects joints. Symptoms generally include joint pain and stiffness. Other symptoms may include redness, warmth, swelling, and decreased range of motion of the affected joints. In some types of arthritis, other organs are also affected. Onset can be gradual or sudden.

Leflunomide, sold under the brand name Arava among others, is an immunosuppressive disease-modifying antirheumatic drug (DMARD), used in active moderate-to-severe rheumatoid arthritis and psoriatic arthritis. It is a pyrimidine synthesis inhibitor that works by inhibiting dihydroorotate dehydrogenase.

Certolizumab pegol, sold under the brand name Cimzia, is a biologic medication for the treatment of Crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.

Belimumab, sold under the brand name Benlysta, is a human monoclonal antibody that inhibits B-cell activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS). It is approved in the United States, Canada, and the European Union to treat systemic lupus erythematosus (SLE).
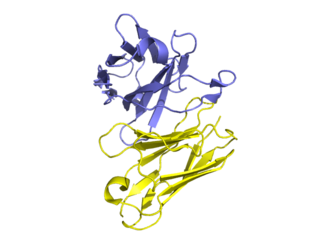
Golimumab is a human monoclonal antibody which is used as an immunosuppressive medication and sold under the brand name Simponi. Golimumab targets tumor necrosis factor alpha (TNF-alpha), a pro-inflammatory molecule and hence is a TNF inhibitor. Profound reduction in C-reactive protein (CRP) levels, interleukin (IL)-6, intercellaular adhesion molecules (ICAM)-1, matrix metalloproteinase (MMP)-3, and vascular endothelial growth factor (VEGF) demonstrates golimumab as an effective modulator of inflammatory markers and bone metabolism.
Toralizumab was a humanized monoclonal antibody and an immunosuppressive drug. Possible indications included treatment of antibody-mediated disorders, T-cell-mediated diseases, and B-cell malignancies such as CLL/small lymphocytic lymphoma, follicular cell lymphoma grade I or II, marginal zone lymphoma, mantle cell lymphoma, MALT lymphoma, Waldenström's macroglobulinemia, monocytoid B-cell lymphoma; relapsed/refractory Hodgkin's disease).

Tolerx, Inc. was a biopharmaceutical company headquartered in Cambridge, Massachusetts. The company was focused on discovering and developing new therapies designed to treat patients by reprogramming the immune system, allowing for long-term remission of immune-related diseases after a short course of therapy. Targeted diseases include type 1 diabetes, rheumatoid arthritis, Inflammatory bowel disease (IBD), cancer, chronic and viral diseases. In 2008, Tolerx was named one of Fierce Biotech’s Fierce 15. In October 2011, Tolerx was shut down due to an unsuccessful Phase III trial in patients recently diagnosed with Type 1 diabetes.
A Janus kinase inhibitor, also known as JAK inhibitor or jakinib, is a type of immune modulating medication, which inhibits the activity of one or more of the Janus kinase family of enzymes, thereby interfering with the JAK-STAT signaling pathway in lymphocytes.
Pegsunercept is a drug for the treatment of rheumatoid arthritis. As of January 2010, Phase II clinical trials have been completed. It is being developed by Amgen.
Blisibimod is a selective antagonist of B-cell activating factor, being developed by Anthera Pharmaceuticals as a treatment for systemic lupus erythematosus. It is currently under active investigation in clinical trials.
Sirukumab is a human monoclonal antibody designed for the treatment of rheumatoid arthritis. It acts against the proinflammatory cytokine Interleukin 6 (IL-6).
Fezakinumab is a human monoclonal antibody against interleukin-22, designed for the treatment of psoriasis and rheumatoid arthritis.
Tabalumab is an anti-B-cell activating factor (BAFF) human monoclonal antibody designed for the treatment of autoimmune diseases and B cell malignancies. Tabalumab was developed by Eli Lilly and Company.
Sarilumab, sold under the brand name Kevzara, is a human monoclonal antibody medication against the interleukin-6 receptor. Regeneron Pharmaceuticals and Sanofi developed the drug for the treatment of rheumatoid arthritis (RA), for which it received US FDA approval on 22 May 2017 and European Medicines Agency approval on 23 June 2017.
Olokizumab is an immunomodulator. It binds to interleukin 6. Hence acting as an Anti-IL-6 therapeutic aimed at inflammatory disease e.g. rheumatoid arthritis (RA).
Ocaratuzumab is a humanized monoclonal antibody designed for the treatment of cancer and autoimmune disorders. The antibody is engineered for enhanced affinity to the CD20 antigen on B-lymphocytes, increased antibody-dependent cell-mediated cytotoxicity (ADCC), and for improved treatment of low-affinity FcγRIIIa allotypes.

Baricitinib, sold under the brand name Olumiant among others, is a medication used for the treatment of rheumatoid arthritis, alopecia areata, and COVID-19. It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2.

Filgotinib, sold under the brand name Jyseleca, is a medication used for the treatment of rheumatoid arthritis (RA). It was developed by the Belgian-Dutch biotech company Galapagos NV.

Upadacitinib, sold under the brand name Rinvoq, is a Janus kinase (JAK) inhibitor medication for the treatment of moderately to severely active rheumatoid arthritis and psoriatic arthritis in adults where methotrexate did not work well or could not be tolerated. Upadacitinib works by blocking the action of enzymes called Janus kinases. These enzymes are involved in setting up processes that lead to inflammation, and blocking their effect brings inflammation in the joints under control.
Ianalumab is a monoclonal antibody that is being investigated for autoimmune hepatitis, multiple sclerosis, pemphigus vulgaris, rheumatoid arthritis, Sjögren syndrome, and systemic lupus erythematosus.









