
In quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function describes an electron's charge distribution around the atom's nucleus, and can be used to calculate the probability of finding an electron in a specific region around the nucleus.

Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized.

In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively.
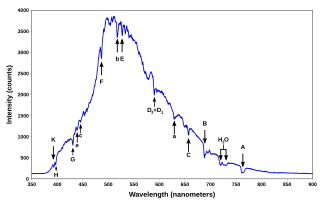
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible.

The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to electrons making a transition from a high energy state to a lower energy state. The photon energy of the emitted photons is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.
In physics, atomic spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. Since unique elements have unique emission spectra, atomic spectroscopy is applied for determination of elemental compositions. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is between optical and mass spectrometry. Mass spectrometry generally gives significantly better analytical performance, but is also significantly more complex. This complexity translates into higher purchase costs, higher operational costs, more operator training, and a greater number of components that can potentially fail. Because optical spectroscopy is often less expensive and has performance adequate for many tasks, it is far more common. Atomic absorption spectrometers are one of the most commonly sold and used analytical devices.
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configuration, the lowest energy term is the one with the greatest value of spin multiplicity. This implies that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs. The rule, discovered by Friedrich Hund in 1925, is of important use in atomic chemistry, spectroscopy, and quantum chemistry, and is often abbreviated to Hund's rule, ignoring Hund's other two rules.

Internal conversion is an atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal conversion, a high-energy electron is emitted from the excited atom, but not from the nucleus. For this reason, the high-speed electrons resulting from internal conversion are not called beta particles, since the latter come from beta decay, where they are newly created in the nuclear decay process.
In quantum mechanics, the principal quantum number is one of four quantum numbers assigned to each electron in an atom to describe that electron's state. Its values are natural numbers making it a discrete variable.
In atomic physics, a magnetic quantum number is a quantum number used to distinguish quantum states of an electron or other particle according to its angular momentum along a given axis in space. The orbital magnetic quantum number distinguishes the orbitals available within a given subshell of an atom. It specifies the component of the orbital angular momentum that lies along a given axis, conventionally called the z-axis, so it describes the orientation of the orbital in space. The spin magnetic quantum numberms specifies the z-axis component of the spin angular momentum for a particle having spin quantum number s. For an electron, s is 1⁄2, and ms is either +1⁄2 or −1⁄2, often called "spin-up" and "spin-down", or α and β. The term magnetic in the name refers to the magnetic dipole moment associated with each type of angular momentum, so states having different magnetic quantum numbers shift in energy in a magnetic field according to the Zeeman effect.
In atomic physics, a term symbol is an abbreviated description of the total spin and orbital angular momentum quantum numbers of the electrons in a multi-electron atom. So while the word symbol suggests otherwise, it represents an actual value of a physical quantity.

In atomic physics and quantum chemistry, the Aufbau principle, also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill subshells of the lowest available energy, then fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. An example is the configuration 1s2 2s2 2p6 3s2 3p3 for the phosphorus atom, meaning that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, and so on.

The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The classification of the series by the Rydberg formula was important in the development of quantum mechanics. The spectral series are important in astronomical spectroscopy for detecting the presence of hydrogen and calculating red shifts.
Core electrons are the electrons in an atom that are not valence electrons and do not participate directly in chemical bonding. The nucleus and the core electrons of an atom form the atomic core. Core electrons are tightly bound to the nucleus. Therefore, unlike valence electrons, core electrons play a secondary role in chemical bonding and reactions by screening the positive charge of the atomic nucleus from the valence electrons.
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on further and further from the nucleus. The shells correspond to the principal quantum numbers (n = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, ...). A useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements represents an electron shell.

Modern spectroscopy in the Western world started in the 17th century. New designs in optics, specifically prisms, enabled systematic observations of the solar spectrum. Isaac Newton first applied the word spectrum to describe the rainbow of colors that combine to form white light. During the early 1800s, Joseph von Fraunhofer conducted experiments with dispersive spectrometers that enabled spectroscopy to become a more precise and quantitative scientific technique. Since then, spectroscopy has played and continues to play a significant role in chemistry, physics and astronomy. Fraunhofer observed and measured dark lines in the Sun's spectrum, which now bear his name although several of them were observed earlier by Wollaston.
The sharp series is a series of spectral lines in the atomic emission spectrum caused when electrons descend from higher-energy s orbitals of an atom to the lowest available p orbital. The spectral lines include some in the visible light, and they extend into the ultraviolet. The lines get closer and closer together as the frequency increases never exceeding the series limit. The sharp series was important in the development of the understanding of electron shells and subshells in atoms. The sharp series has given the letter s to the s atomic orbital or subshell.

The diffuse series is a series of spectral lines in the atomic emission spectrum caused when electrons jump between the lowest p orbital and d orbitals of an atom. The total orbital angular momentum changes between 1 and 2. The spectral lines include some in the visible light, and may extend into ultraviolet or near infrared. The lines get closer and closer together as the frequency increases never exceeding the series limit. The diffuse series was important in the development of the understanding of electron shells and subshells in atoms. The diffuse series has given the letter d to the d atomic orbital or subshell.
The fundamental series is a set of spectral lines in a set caused by transition between d and f orbitals in atoms.











