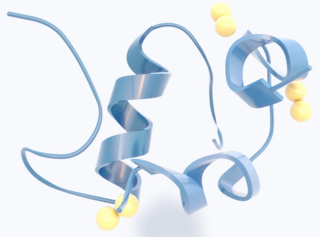
Insulin is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (INS) gene. It is the main anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats, and protein by promoting the absorption of glucose from the blood into cells of the liver, fat, and skeletal muscles. In these tissues the absorbed glucose is converted into either glycogen, via glycogenesis, or fats (triglycerides), via lipogenesis; in the liver, glucose is converted into both. Glucose production and secretion by the liver are strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is thus an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules in the cells. Low insulin in the blood has the opposite effect, promoting widespread catabolism, especially of reserve body fat.

Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.

Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion.

Beta cells (β-cells) are specialized endocrine cells located within the pancreatic islets of Langerhans responsible for the production and release of insulin and amylin. Constituting ~50–70% of cells in human islets, beta cells play a vital role in maintaining blood glucose levels. Problems with beta cells can lead to disorders such as diabetes.

In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones.

The connecting peptide, or C-peptide, is a short 31-amino-acid polypeptide that connects insulin's A-chain to its B-chain in the proinsulin molecule. In the context of diabetes or hypoglycemia, a measurement of C-peptide blood serum levels can be used to distinguish between different conditions with similar clinical features.

Protein disulfide isomerase, or PDI, is an enzyme in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore the enzyme acts to catalyze protein folding.

The insulin receptor (IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase. Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis; a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes and cancer. Insulin signalling controls access to blood glucose in body cells. When insulin falls, especially in those with high insulin sensitivity, body cells begin only to have access to lipids that do not require transport across the membrane. So, in this way, insulin is the key regulator of fat metabolism as well. Biochemically, the insulin receptor is encoded by a single gene INSR, from which alternate splicing during transcription results in either IR-A or IR-B isoforms. Downstream post-translational events of either isoform result in the formation of a proteolytically cleaved α and β subunit, which upon combination are ultimately capable of homo or hetero-dimerisation to produce the ≈320 kDa disulfide-linked transmembrane insulin receptor.
Gonadotropins are glycoprotein hormones secreted by gonadotropic cells of the anterior pituitary of vertebrates. This family includes the mammalian hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH), the placental/chorionic gonadotropins, human chorionic gonadotropin (hCG) and equine chorionic gonadotropin (eCG), as well as at least two forms of fish gonadotropins. These hormones are central to the complex endocrine system that regulates normal growth, sexual development, and reproductive function. LH and FSH are secreted by the anterior pituitary gland, while hCG and eCG are secreted by the placenta in pregnant women and mares, respectively. The gonadotropins act on the gonads, controlling gamete and sex hormone production.

Resistin also known as adipose tissue-specific secretory factor (ADSF) or C/EBP-epsilon-regulated myeloid-specific secreted cysteine-rich protein (XCP1) is a cysteine-rich peptide hormone derived from adipose tissue that in humans is encoded by the RETN gene.

Amylin, or islet amyloid polypeptide (IAPP), is a 37-residue peptide hormone. It is co-secreted with insulin from the pancreatic β-cells in the ratio of approximately 100:1 (insulin:amylin). Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.

Proprotein convertase 1, also known as prohormone convertase, prohormone convertase 3, or neuroendocrine convertase 1 and often abbreviated as PC1/3 is an enzyme that in humans is encoded by the PCSK1 gene. PCSK1 and PCSK2 differentially cleave proopiomelanocortin and they act together to process proinsulin and proglucagon in pancreatic islets.
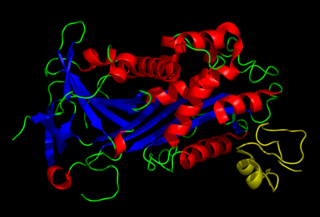
Vitronectin is a glycoprotein of the hemopexin family which is synthesized and excreted by the liver, and abundantly found in serum, the extracellular matrix and bone. In humans it is encoded by the VTN gene.
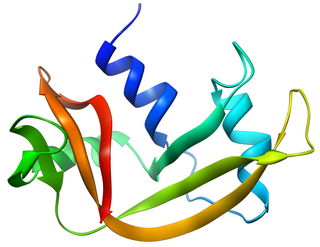
Bovine pancreatic ribonuclease, also often referred to as bovine pancreatic ribonuclease A or simply RNase A, is a pancreatic ribonuclease enzyme that cleaves single-stranded RNA. Bovine pancreatic ribonuclease is one of the classic model systems of protein science. Two Nobel Prizes in Chemistry have been awarded in recognition of work on bovine pancreatic ribonuclease: in 1972, the Prize was awarded to Christian Anfinsen for his work on protein folding and to Stanford Moore and William Stein for their work on the relationship between the protein's structure and its chemical mechanism; in 1984, the Prize was awarded to Robert Bruce Merrifield for development of chemical synthesis of proteins.
Proprotein convertases (PPCs) are a family of proteins that activate other proteins. Many proteins are inactive when they are first synthesized, because they contain chains of amino acids that block their activity. Proprotein convertases remove those chains and activate the protein. The prototypical proprotein convertase is furin. Proprotein convertases have medical significance, because they are involved in many important biological processes, such as cholesterol synthesis. Compounds called proprotein convertase inhibitors can block their action, and block the target proteins from becoming active. Many proprotein convertases, especially furin and PACE4, are involved in pathological processes such as viral infection, inflammation, hypercholesterolemia, and cancer, and have been postulated as therapeutic targets for some of these diseases.

Proprotein convertase 2 (PC2) also known as prohormone convertase 2 or neuroendocrine convertase 2 (NEC2) is a serine protease and proprotein convertase PC2, like proprotein convertase 1 (PC1), is an enzyme responsible for the first step in the maturation of many neuroendocrine peptides from their precursors, such as the conversion of proinsulin to insulin intermediates. To generate the bioactive form of insulin, a second step involving the removal of C-terminal basic residues is required; this step is mediated by carboxypeptidases E and/or D. PC2 plays only a minor role in the first step of insulin biosynthesis, but a greater role in the first step of glucagon biosynthesis compared to PC1. PC2 binds to the neuroendocrine protein named 7B2, and if this protein is not present, proPC2 cannot become enzymatically active. 7B2 accomplishes this by preventing the aggregation of proPC2 to inactivatable forms. The C-terminal domain of 7B2 also inhibits PC2 activity until it is cleaved into smaller inactive forms that lack carboxy-terminal basic residues. Thus, 7B2 is both an activator and an inhibitor of PC2. PC2 has been identified in a number of animals, including C. elegans.

Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in alpha-amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents. Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.

The insulin/IGF/relaxin family is a group of evolutionary related proteins which possess a variety of hormonal activities. Family members in human include two subfamilies:
The insulin transduction pathway is a biochemical pathway by which insulin increases the uptake of glucose into fat and muscle cells and reduces the synthesis of glucose in the liver and hence is involved in maintaining glucose homeostasis. This pathway is also influenced by fed versus fasting states, stress levels, and a variety of other hormones.
A prohormone is a committed precursor of a hormone consisting of peptide hormones synthesized together that has a minimal hormonal effect by itself because of its expression-suppressing structure, often created by protein folding and binding additional peptide chains to certain ends, that makes hormone receptor binding sites located on its peptide hormone chain segments inaccessible. Prohormones can travel the blood stream as a hormone in an inactivated form, ready to be activated later in the cell by post-translational modification.




















