
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.

Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known.
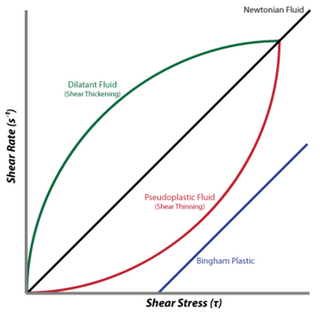
A dilatant material is one in which viscosity increases with the rate of shear strain. Such a shear thickening fluid, also known by the initialism STF, is an example of a non-Newtonian fluid. This behaviour is usually not observed in pure materials, but can occur in suspensions.
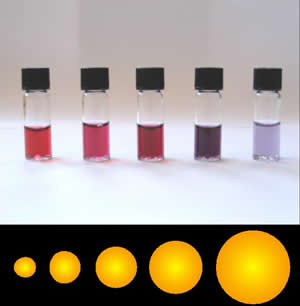
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red or blue-purple . Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine.
The DLVO theory explains the aggregation and kinetic stability of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium. It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions. The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale, . For two spheres of radius each having a charge separated by a center-to-center distance in a fluid of dielectric constant containing a concentration of monovalent ions, the electrostatic potential takes the form of a screened-Coulomb or Yukawa potential,

In colloidal chemistry, flocculation is a process by which colloidal particles come out of suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion and are not truly dissolved in solution.

Froth flotation is a process for selectively separating hydrophobic materials from hydrophilic. This is used in mineral processing, paper recycling and waste-water treatment industries. Historically this was first used in the mining industry, where it was one of the great enabling technologies of the 20th century. It has been described as "the single most important operation used for the recovery and upgrading of sulfide ores". The development of froth flotation has improved the recovery of valuable minerals, such as copper- and lead-bearing minerals. Along with mechanized mining, it has allowed the economic recovery of valuable metals from much lower-grade ore than previously.

Particle agglomeration refers to the formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid phase stick to each other, and spontaneously form irregular particle assemblages, flocs, or agglomerates. This phenomenon is also referred to as coagulation or flocculation and such a suspension is also called unstable. Particle agglomeration can be induced by adding salts or other chemicals referred to as coagulant or flocculant.

Diffusiophoresis is the spontaneous motion of colloidal particles or molecules in a fluid, induced by a concentration gradient of a different substance. In other words, it is motion of one species, A, in response to a concentration gradient in another species, B. Typically, A is colloidal particles which are in aqueous solution in which B is a dissolved salt such as sodium chloride, and so the particles of A are much larger than the ions of B. But both A and B could be polymer molecules, and B could be a small molecule. For example, concentration gradients in ethanol solutions in water move 1 μm diameter colloidal particles with diffusiophoretic velocities of order 0.1 to 1 μm/s, the movement is towards regions of the solution with lower ethanol concentration. Both species A and B will typically be diffusing but diffusiophoresis is distinct from simple diffusion: in simple diffusion a species A moves down a gradient in its own concentration.
A Ramsden emulsion, sometimes named Pickering emulsion, is an emulsion that is stabilized by solid particles which adsorb onto the interface between the water and oil phases. Typically, the emulsions are either water-in-oil or oil-in-water emulsions, but other more complex systems such as water-in-water, oil-in-oil, water-in-oil-in-water, and oil-in-water-in-oil also do exist. Pickering emulsions were named after S.U. Pickering, who described the phenomenon in 1907, although the effect was first recognized by Walter Ramsden in 1903.
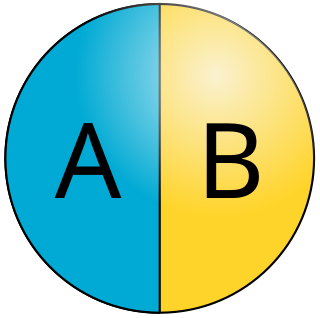
Janus particles are special types of nanoparticles or microparticles whose surfaces have two or more distinct physical properties. This unique surface of Janus particles allows two different types of chemistry to occur on the same particle. The simplest case of a Janus particle is achieved by dividing the particle into two distinct parts, each of them either made of a different material, or bearing different functional groups. For example, a Janus particle may have one half of its surface composed of hydrophilic groups and the other half hydrophobic groups, the particles might have two surfaces of different color, fluorescence, or magnetic properties. This gives these particles unique properties related to their asymmetric structure and/or functionalization.
Hydrophobic silica is a form of silicon dioxide that has hydrophobic groups chemically bonded to the surface. The hydrophobic groups are normally alkyl or polydimethylsiloxane chains. Hydrophobic silica can be processed in different ways; such as fumed silica, precipitated silica, and aerosol assisted self assembly, all existing in the form of nanoparticles.
The gold number is the minimum weight of a protective colloid required to prevent the coagulation of 10 ml of a standard hydro gold sol when 1 ml of a 10% sodium chloride solution is added to it. It was first used by Richard Adolf Zsigmondy in 1901.
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.
Adsorption of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.
The Stöber process is a chemical process used to prepare silica particles of controllable and uniform size for applications in materials science. It was pioneering when it was reported by Werner Stöber and his team in 1968, and remains today the most widely used wet chemistry synthetic approach to silica nanoparticles. It is an example of a sol-gel process wherein a molecular precursor is first reacted with water in an alcoholic solution, the resulting molecules then joining together to build larger structures. The reaction produces silica particles with diameters ranging from 50 to 2000 nm, depending on conditions. The process has been actively researched since its discovery, including efforts to understand its kinetics and mechanism – a particle aggregation model was found to be a better fit for the experimental data than the initially hypothesized LaMer model. The newly acquired understanding has enabled researchers to exert a high degree of control over particle size and distribution and to fine-tune the physical properties of the resulting material in order to suit intended applications.

Bovine submaxillary mucin (BSM) coatings are a surface treatment provided to biomaterials intended to reduce the growth of disadvantageous bacteria and fungi such as S. epidermidis, E. coli, and Candida albicans. BSM is a substance extracted from the fresh salivary glands of cows. It exhibits unique physical properties, such as high molecular weight and amphiphilicity, that allow it to be used for many biomedical applications.

Sour cream is a dairy product obtained by fermenting regular cream with certain kinds of lactic acid bacteria. The bacterial culture, which is introduced either deliberately or naturally, sours and thickens the cream. Its name comes from the production of lactic acid by bacterial fermentation, which is called souring. Crème fraîche is one type of sour cream with a high fat content and less sour taste.
Polymer soil stabilization refers to the addition of polymers to improve the physical properties of soils, most often for geotechnical engineering, construction, or agricultural projects. Even at very small concentrations within soils, various polymers have been shown to increase water retention and reduce erosion, increase soil shear strength, and support soil structure. A wide range of polymers have been used to address problems ranging from the prevention of desertification to the reinforcement of roadbeds.
A protein corona is a dynamic coating of biomolecules, usually proteins, around the surface of a nanoparticle that forms spontaneously in colloidal nanomaterials upon exposure to biological mediums. Protein coronas can form in many different patterns depending on their size, shape, composition, charge, and surface functional groups, and have properties that vary in different environmental factors like temperature, pH, shearing stress, immersed media composition, and exposing time. These coatings are also changeable according to the conditions of the biochemical and physiochemical surface interactions. Types of protein coronas are known to be divided into two categories: “hard” and “soft”. “Hard” coronas have higher-affinity proteins that are irreversibly bonded to the nanoparticle surface, while “soft” coronas have lower-affinity proteins on the nanoparticle surface that are reversibly bound. These reversibly-bound proteins allow for the biomolecules in “soft” protein coronas to be exchanged or detached over time for various applications. This process is governed by the intermolecular protein-nanoparticle and protein-protein interactions that exist within a solution. In "soft" protein coronas, it is common to observe an exchange of proteins at the surface; larger proteins with lower affinities will often aggregate to the surface of the nanoparticle first, and over time, smaller proteins with higher affinities will replace them, "hardening" the corona, known as the Vroman effect.











