
Ragweeds are flowering plants in the genus Ambrosia in the aster family, Asteraceae. They are distributed in the tropical and subtropical regions of the Americas, especially North America, where the origin and center of diversity of the genus are in the southwestern United States and northwestern Mexico. Several species have been introduced to the Old World and some have naturalized and have become invasive species. Ragweed species are expected to continue spreading across Europe in the near future in response to ongoing climate change.

Thymol (also known as 2-isopropyl-5-methylphenol, IPMP) is a natural monoterpenoid phenol derivative of p-Cymene, C10H14O, isomeric with carvacrol, found in oil of thyme, and extracted from Thymus vulgaris (common thyme), Ajwain and various other kinds of plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties. Thymol also provides the distinctive, strong flavor of the culinary herb thyme, also produced from T. vulgaris.

Quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure NR+
4, R being an alkyl group or an aryl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.
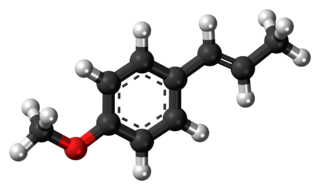
Anethole is an organic compound that is widely used as a flavoring substance. It is a derivative of phenylpropene, a type of aromatic compound that occurs widely in nature, in essential oils. It is in the class of Phenylpropanoid organic compounds. It contributes a large component of the odor and flavor of anise and fennel, anise myrtle (Myrtaceae), liquorice (Fabaceae), magnolia blossoms, and star anise (Schisandraceae). Closely related to anethole is its isomer estragole, abundant in tarragon (Asteraceae) and basil (Lamiaceae), that has a flavor reminiscent of anise. It is a colorless, fragrant, mildly volatile liquid. Anethole is only slightly soluble in water but exhibits high solubility in ethanol. This trait causes certain anise-flavored liqueurs to become opaque when diluted with water, the ouzo effect.

An ionophore is a chemical species that reversibly binds ions. Many ionophores are lipid-soluble entities that transport ions across the cell membrane. Ionophores catalyze ion transport across hydrophobic membranes, such as liquid polymeric membranes or lipid bilayers found in the living cells or synthetic vesicles (liposomes). Structurally, an ionophore contains a hydrophilic center and a hydrophobic portion that interacts with the membrane.

Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct antimicrobial activity, immune signalling activities, or both. They are variously active against bacteria, fungi and many enveloped and nonenveloped viruses. They are typically 18-45 amino acids in length, with three or four highly conserved disulphide bonds.

Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus Streptomyces. It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It is a structural analogue of 2-C-methyl-D-erythrose 4-phosphate. It inhibits the E. coli enzyme with a KI value of 38 nM (4), MTB at 80 nM, and the Francisella enzyme at 99 nM. Several mutations in the E. coli DXP reductoisomerase were found to confer resistance to fosmidomycin.

Eucalyptol is monoterpenoid. A colorless liquid, it is a bicyclic ether. Eucalyptol has a fresh mint-like smell and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up 90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification.

Novobiocin, also known as albamycin or cathomycin, is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the order Actinobacteria. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.

Caryophyllene, more formally (−)-β-caryophyllene, is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, rosemary, and hops. It is usually found as a mixture with isocaryophyllene and α-humulene, a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature.
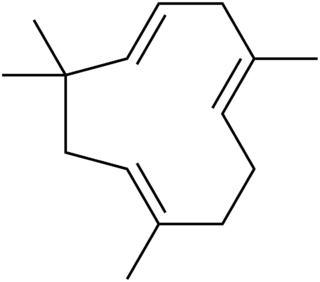
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C=C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of Humulus lupulus (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.
The name Catuaba is used for the infusions of the bark of a number of trees native to Brazil. The most widely used barks are derived from the trees Trichilia catigua and Erythroxylum vaccinifolium. Other catuaba preparations use the bark of trees from the following genera or families: Anemopaegma, Ilex, Micropholis, Phyllanthus, Secondatia, Tetragastris and species from the Myrtaceae.

Miltefosine, sold under the trade name Impavido among others, is a medication mainly used to treat leishmaniasis and free-living amoeba infections such as Naegleria fowleri and Balamuthia mandrillaris. This includes the three forms of leishmaniasis: cutaneous, visceral and mucosal. It may be used with liposomal amphotericin B or paromomycin. It is taken by mouth.

Polygodial is chemical compound found in dorrigo pepper, mountain pepper, horopito, canelo, paracress, water-pepper, and Dendrodoris limbata.

Gallium maltolate is a coordination complex consisting of a trivalent gallium cation coordinated to three maltolate ligands. The compound is a potential therapeutic agent for cancer, infectious disease, and inflammatory disease. A cosmetic skin cream containing gallium maltolate is marketed under the name Gallixa. It is a colorless solid with significant solubility in both water and lipids.

Flumequine is a synthetic fluoroquinolone antibiotic used to treat bacterial infections. It is a first-generation fluoroquinolone antibacterial that has been removed from clinical use and is no longer being marketed. The marketing authorization of flumequine has been suspended throughout the EU. It kills bacteria by interfering with the enzymes that cause DNA to unwind and duplicate. Flumequine was used in veterinarian medicine for the treatment of enteric infections, as well as to treat cattle, swine, chickens, and fish, but only in a limited number of countries. It was occasionally used in France to treat urinary tract infections under the trade name Apurone. However this was a limited indication because only minimal serum levels were achieved.

Ocimum gratissimum, also known as clove basil, African basil, and in Hawaii as wild basil, is a species of Ocimum. It is native to Africa, Madagascar, southern Asia, and the Bismarck Archipelago, and naturalized in Polynesia, Hawaii, Mexico, Panama, West Indies, Brazil, and Bolivia.

α-Cadinol or 10α-hydroxy-4-cadinene is an organic compound, a sesquiterpenoid alcohol.

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of pomegranates.
Cynaropicrin is a sesquiterpene lactone of the guaianolide type found mainly in leaves of artichoke plants. It is one of the compounds that gives the artichoke its characteristic bitterness. It is found in artichoke leaves with an abundance of approximately 87 g/kg, but can hardly be found in other parts of the plant. Cynaropicrin makes up about 0.7% of leaf extracts of the artichoke. It exhibits a large diversity of bioactivities and shows properties such as anti-inflammatory, antifeedant and activation of bitter sensory receptors, but has not yet been used in medicine. Despite its pharmacologically beneficial properties, it can be toxic in higher doses. The compound has attracted attention in recent years as a potential anticancer drug.




















