A coiled coil is a structural motif in proteins in which 2–7 alpha-helices are coiled together like the strands of a rope. They have been found in roughly 5-10% of proteins and have a variety of functions. They are one of the most widespread motifs found in protein-protein interactions. To aid protein study, several tools have been developed to predict coiled-coils in protein structures. Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin.
Opsonins are extracellular proteins that, when bound to substances or cells, induce phagocytes to phagocytose the substances or cells with the opsonins bound. Thus, opsonins act as tags to label things in the body that should be phagocytosed by phagocytes. Different types of things ("targets") can be tagged by opsonins for phagocytosis, including: pathogens, cancer cells, aged cells, dead or dying cells, excess synapses, or protein aggregates. Opsonins help clear pathogens, as well as dead, dying and diseased cells.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils, as well as by epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein, with the defining property being the ability to bind and remove modified low density lipoproteins (LDL). Today scavenger receptors are known to be involved in a wide range of processes, such as: homeostasis, apoptosis, inflammatory diseases and pathogen clearance. Scavenger receptors are mainly found on myeloid cells and other cells that bind to numerous ligands, primarily endogenous and modified host-molecules together with pathogen-associated molecular patterns(PAMPs), and remove them. The Kupffer cells in the liver are particularly rich in scavenger receptors, includes SR-A I, SR-A II, and MARCO.

Pulmonary surfactant is a surface-active complex of phospholipids and proteins formed by type II alveolar cells. The proteins and lipids that make up the surfactant have both hydrophilic and hydrophobic regions. By adsorbing to the air-water interface of alveoli, with hydrophilic head groups in the water and the hydrophobic tails facing towards the air, the main lipid component of surfactant, dipalmitoylphosphatidylcholine (DPPC), reduces surface tension.
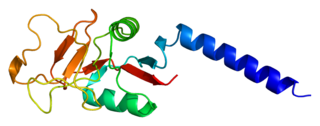
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the COL3A1 gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain.
Collectins (collagen-containing C-type lectins) are a part of the innate immune system. They form a family of collagenous Ca2+-dependent defense lectins, which are found in animals. Collectins are soluble pattern recognition receptors (PRRs). Their function is to bind to oligosaccharide structure or lipids that are on the surface of microorganisms. Like other PRRs they bind pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) of oligosaccharide origin. Binding of collectins to microorganisms may trigger elimination of microorganisms by aggregation, complement activation, opsonization, activation of phagocytosis, or inhibition of microbial growth. Other functions of collectins are modulation of inflammatory, allergic responses, adaptive immune system and clearance of apoptotic cells.
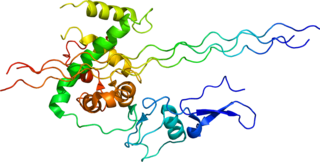
Mannose-binding lectin (MBL), also called mannan-binding lectin or mannan-binding protein (MBP), is a lectin that is instrumental in innate immunity as an opsonin and via the lectin pathway.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).

Surfactant protein D, also known as SP-D, is a lung surfactant protein part of the collagenous family of lectins called collectin. In humans, SP-D is encoded by the SFTPD gene and is part of the innate immune system. Each SP-D subunit is composed of an N-terminal domain, a collagenous region, a nucleating neck region, and a C-terminal lectin domain. Three of these subunits assemble to form a homotrimer, which further assemble into a tetrameric complex.
Surfactant protein A is an innate immune system collectin. It is water-soluble and has collagen-like domains similar to SP-D. It is part of the innate immune system and is used to opsonize bacterial cells in the alveoli marking them for phagocytosis by alveolar macrophages. SP-A may also play a role in negative feedback limiting the secretion of pulmonary surfactant. SP-A is not required for pulmonary surfactant to function but does confer immune effects to the organism.

Surfactant protein B is an essential lipid-associated protein found in pulmonary surfactant. Without it, the lung would not be able to inflate after a deep breath out. It rearranges lipid molecules in the fluid lining the lung so that tiny air sacs in the lung, called alveoli, can more easily inflate.

Surfactant protein C (SP-C), is one of the pulmonary surfactant proteins. In humans this is encoded by the SFTPC gene.

Surfactant protein A1(SP-A1), also known as Pulmonary surfactant-associated protein A1(PSP-A) is a protein that in humans is encoded by the SFTPA1 gene.

Ficolin-1, and also commonly termed M-ficolin is a protein that in humans is encoded by the FCN1 gene.

Surfactant protein A2(SP-A2), also known as Pulmonary surfactant-associated protein A2(PSP-A2) is a protein that in humans is encoded by the SFTPA2 gene.
Ficolins are pattern recognition receptors that bind to acetyl groups present in the carbohydrates of bacterial surfaces and mediate activation of the lectin pathway of the complement cascade.

In molecular biology, trimeric autotransporter adhesins (TAAs), are proteins found on the outer membrane of Gram-negative bacteria. Bacteria use TAAs in order to infect their host cells via a process called cell adhesion. TAAs also go by another name, oligomeric coiled-coil adhesins, which is shortened to OCAs. In essence, they are virulence factors, factors that make the bacteria harmful and infective to the host organism.

In molecular biology, YadA is a protein domain which is short for Yersinia adhesin A. These proteins have strong sequence and structural homology, particularly at their C-terminal end. The function is to promote their pathogenicity and virulence in host cells, though cell adhesion. YadA is found in three pathogenic species of Yersinia, Y. pestis,Y. pseudotuberculosis, and Y. enterocolitica. The YadA domain is encoded for by a virulence plasmid in Yersinia, which encodes a type-III secretion (T3S) system consisting of the Ysc injectisome and the Yop effectors.
Erika Crouch is a professor of pathology and the Carol B. and Jerome T. Loeb Professor of Medical Education at Washington University in St. Louis.










