Related Research Articles

Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium.

A flare, also sometimes called a fusée, fusee, or bengala in some Latin-speaking countries, is a type of pyrotechnic that produces a bright light or intense heat without an explosion. Flares are used for distress signaling, illumination, or defensive countermeasures in civilian and military applications. Flares may be ground pyrotechnics, projectile pyrotechnics, or parachute-suspended to provide maximum illumination time over a large area. Projectile pyrotechnics may be dropped from aircraft, fired from rocket or artillery, or deployed by flare guns or handheld percussive tubes.

Thermite is a pyrotechnic composition of metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties are not explosive, but can create brief bursts of heat and high temperature in a small area. Its form of action is similar to that of other fuel-oxidizer mixtures, such as black powder.

Pyrotechnics is the science and craft of creating such things as fireworks, safety matches, oxygen candles, explosive bolts and other fasteners, parts of automotive airbags, as well as gas-pressure blasting in mining, quarrying, and demolition. This trade relies upon self-contained and self-sustained exothermic chemical reactions to make heat, light, gas, smoke and/or sound. The name comes from the Greek words pyr ("fire") and tekhnikos.

A flame is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they are then considered plasma.

Tracer ammunition (AMO) (Tracers) are bullets or cannon-caliber projectiles that are built with a small pyrotechnic charge in their base. When fired, the pyrotechnic composition is ignited by the burning powder and burns very brightly, making the projectile trajectory visible to the naked eye during daylight, and very bright during nighttime firing. This allows the shooter to visually trace the flight path of the projectile and thus make necessary ballistic corrections, without having to confirm projectile impacts and without even using the sights of the weapon. Tracer fire can also be used as a marking tool to signal other shooters to concentrate their fire on a particular target during battle.

Magnesium diboride is the inorganic compound with the formula MgB2. It is a dark gray, water-insoluble solid. The compound has attracted attention because it becomes superconducting at 39 K (−234 °C). In terms of its composition, MgB2 differs strikingly from most low-temperature superconductors, which feature mainly transition metals. Its superconducting mechanism is primarily described by BCS theory.

Flash powder is a pyrotechnic composition, a mixture of oxidizer and metallic fuel, which burns quickly and produces a loud noise regardless of confinement. It is widely used in theatrical pyrotechnics and fireworks and was once used for flashes in photography.
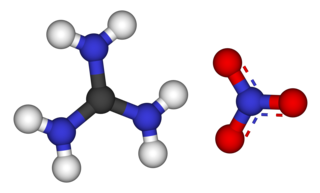
Guanidine nitrate is the chemical compound with the formula [C(NH2)3]NO3. It is a colorless, water-soluble salt. It is produced on a large scale and finds use as precursor for nitroguanidine, fuel in pyrotechnics and gas generators. Its correct name is guanidinium nitrate, but the colloquial term guanidine nitrate is widely used.
A pyrotechnic colorant is a chemical compound which causes a flame to burn with a particular color. These are used to create the colors in pyrotechnic compositions like fireworks and colored fires. The color-producing species are usually created from other chemicals during the reaction. Metal salts are commonly used; elemental metals are used rarely.
Nano-thermite or super-thermite is a metastable intermolecular composite (MIC) characterized by a particle size of its main constituents, a metal and a metal oxide, under 100 nanometers. This allows for high and customizable reaction rates. Nano-thermites contain an oxidizer and a reducing agent, which are intimately mixed on the nanometer scale. MICs, including nano-thermitic materials, are a type of reactive materials investigated for military use, as well as for general applications involving propellants, explosives, and pyrotechnics.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
Magnesium/Teflon/Viton (MTV) is a pyrolant. Teflon and Viton are trademarks of DuPont for polytetrafluoroethylene, (C2F4)n, and fluoroelastomer, (CH2CF2)n(CF(CF3)CF2)n.

Copper(I) bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds and as a lasing medium in copper bromide lasers.

Barium oxalate (BaC2O4), a barium salt of oxalic acid, is a white odorless powder that is sometimes used as a green pyrotechnic colorant generally in specialized pyrotechnic compositions containing magnesium metal powder. Flame color is rich and vivid without additional chlorine donors. Such compositions burn rate is satisfied without commonly used oxidizers as nitrates, chlorates and perchlorates.

Calcium disilicide (CaSi2) is an inorganic compound, a silicide of calcium. It is a whitish or dark grey to black solid matter with melting point 1033 °C. It is insoluble in water, but may decompose when subjected to moisture, evolving hydrogen and producing calcium hydroxide. It decomposes in hot water, and is flammable and may ignite spontaneously in air.
Ammonium perchlorate composite propellant (APCP) is a solid-propellant rocket fuel. It differs from many traditional solid rocket propellants such as black powder or zinc-sulfur, not only in chemical composition and overall performance but also by the nature of how it is processed. APCP is cast into shape, as opposed to powder pressing as with black powder. This provides manufacturing regularity and repeatability, which are necessary requirements for use in the aerospace industry.

A flare or decoy flare is an aerial infrared countermeasure used by a plane or helicopter to counter an infrared homing ("heat-seeking") surface-to-air missile or air-to-air missile. Flares are commonly composed of a pyrotechnic composition based on magnesium or another hot-burning metal, with burning temperature equal to or hotter than engine exhaust. The aim is to make the infrared-guided missile seek out the heat signature from the flare rather than the aircraft's engines.
In pyrotechnics, a pyrotechnic initiator is a device containing a pyrotechnic composition used primarily to ignite other, more difficult-to-ignite materials, such as thermites, gas generators, and solid-fuel rockets. The name is often used also for the compositions themselves.
An environmentally-friendly red-light flare was a pyrotechnic (firework) flare which used lithium-based formulations that emitted red light. A flare is used for signaling, illumination, or defensive countermeasures in civilian or military applications. It is based on a non-hygroscopic dilithium nitrogen-rich salt that served as an oxidizer and red colorant. The U.S. Army Research Laboratory and the Ludwig Maximilian Institution were credited as the research facilities for developing this product announced in January 2018.
References
- ↑ T. Kuwahara, T. Ochiachi, Burning Rate of Mg/TF Pyrolants, Proceedings of the 18th Int. Pyrotechnics Seminar, 1992, p. 539.
- ↑ H. Ellern, Modern Pyrotechnics, CRC Press, 1968, p. 221ff.
- ↑ J. H. McLain, Pyrotechnics, The Franklin Institute Press, Philadelphia, 1980, pp 22 ff.
- ↑ T. Kuwahara, T. Kohno, C. H. Wang, Static Electric Sensitivity Characteristics of Zr/, Pyrolants, Prop., Explos., Pyrotech. 292004, 56.
- ↑ [ dead link ] J. R. Ward, L. J. Decker, A. W. Barrows, Burning Rates of Pressed Strands of a Stoichiometric Magnesium-Sodium Nitrate Mix, Combust. Flame 511983, 121.
- ↑ E.-C. Koch Metal/Fluorocarbon Pyrolants: VI. Combustion Behaviour and Radiation Properties of Magnesium/Poly(Carbon Monofluoride) Pyrolant, Prop., Explos., Pyrotech. 302005 209.
