
Steel is an alloy of iron and carbon with improved strength and fracture resistance compared to other forms of iron. Because of its high tensile strength and low cost, steel is one of the most commonly manufactured materials in the world. Steel is used in buildings, as concrete reinforcing rods, in bridges, infrastructure, tools, ships, trains, cars, bicycles, machines, electrical appliances, furniture, and weapons.

Heat treating is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.

Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation.

Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902). It exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures.

Bainite is a plate-like microstructure that forms in steels at temperatures of 125–550 °C. First described by E. S. Davenport and Edgar Bain, it is one of the products that may form when austenite is cooled past a temperature where it is no longer thermodynamically stable with respect to ferrite, cementite, or ferrite and cementite. Davenport and Bain originally described the microstructure as being similar in appearance to tempered martensite.
In metallurgy, a shape-memory alloy (SMA) is an alloy that can be deformed when cold but returns to its pre-deformed ("remembered") shape when heated. It is also known in other names such as memory metal, memory alloy, smart metal, smart alloy, and muscle wire. The "memorized geometry" can be modified by fixating the desired geometry and subjecting it to a thermal treatment, for example a wire can be taught to memorize the shape of a coil spring.
Magnetic shape memory alloys (MSMAs), also called ferromagnetic shape memory alloys (FSMA), are particular shape memory alloys which produce forces and deformations in response to a magnetic field. The thermal shape memory effect has been obtained in these materials, too.

Maraging steels are steels that are known for possessing superior strength and toughness without losing ductility. Aging refers to the extended heat-treatment process. These steels are a special class of very-low-carbon ultra-high-strength steels that derive their strength not from carbon, but from precipitation of intermetallic compounds. The principal alloying element is 15 to 25 wt% nickel. Secondary alloying elements, which include cobalt, molybdenum and titanium, are added to produce intermetallic precipitates. Original development was carried out on 20 and 25 wt% Ni steels to which small additions of aluminium, titanium, and niobium were made; a rise in the price of cobalt in the late 1970s led to the development of cobalt-free maraging steels.

Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered at much higher temperatures.
Cryogenic hardening is a cryogenic treatment process where the material is cooled to approximately −185 °C (−301 °F), usually using liquid nitrogen. It can have a profound effect on the mechanical properties of certain steels, provided their composition and prior heat treatment are such that they retain some austenite at room temperature. It is designed to increase the amount of martensite in the steel's crystal structure, increasing its strength and hardness, sometimes at the cost of toughness. Presently this treatment is being used on tool steels, high-carbon, high-chromium steels and in some cases to cemented carbide to obtain excellent wear resistance. Recent research shows that there is precipitation of fine carbides in the matrix during this treatment which imparts very high wear resistance to the steels.
Hardening is a metallurgical metalworking process used to increase the hardness of a metal. The hardness of a metal is directly proportional to the uniaxial yield stress at the location of the imposed strain. A harder metal will have a higher resistance to plastic deformation than a less hard metal.
An archwire in orthodontics is a wire conforming to the alveolar or dental arch that can be used with dental braces as a source of force in correcting irregularities in the position of the teeth. An archwire can also be used to maintain existing dental positions; in this case it has a retentive purpose.
In metallurgy and materials science, annealing is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for an appropriate amount of time and then cooling.
Pseudoelasticity, sometimes called superelasticity, is an elastic (reversible) response to an applied stress, caused by a phase transformation between the austenitic and martensitic phases of a crystal. It is exhibited in shape-memory alloys.

Nickel titanium, also known as nitinol, is a metal alloy of nickel and titanium, where the two elements are present in roughly equal atomic percentages. Different alloys are named according to the weight percentage of nickel; e.g., nitinol 55 and nitinol 60.
Ferroelasticity is a phenomenon in which a material may exhibit a spontaneous strain. Usually, a crystal has two or more stable orientational states in the absence of mechanical stress or electric field, i.e. remanent states, and can be reproducibly switched between states by the application of mechanical stress. In ferroics, ferroelasticity is the mechanical equivalent of ferroelectricity and ferromagnetism. When stress is applied to a ferroelastic material, a phase change will occur in the material from one phase to an equally stable phase, either of different crystal structure, or of different orientation. This stress-induced phase change results in a spontaneous strain in the material.
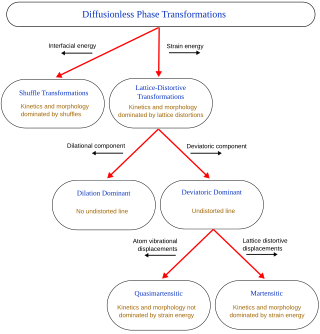
A diffusionless transformation, commonly known as displacive transformation, denote solid-state alterations in the crystal structure that do not hinge on the diffusion of atoms across extensive distances. Rather, these transformations manifest as a result of synchronized shifts in atomic positions, wherein atoms undergo displacements of distances smaller than the spacing between adjacent atoms, all while preserving their relative arrangement. An exemplar of such a phenomenon is the martensitic transformation, a notable occurrence observed in the context of steel materials. The term "martensite" was originally coined to describe the rigid and finely dispersed constituent that emerges in steels subjected to rapid cooling. Subsequent investigations revealed that materials beyond ferrous alloys, such as non-ferrous alloys and ceramics, can also undergo diffusionless transformations. Consequently, the term "martensite" has evolved to encompass the resultant product arising from such transformations in a more inclusive manner. In the context of diffusionless transformations, a cooperative and homogeneous movement occurs, leading to a modification in the crystal structure during a phase change. These movements are small, usually less than their interatomic distances, and the neighbors of an atom remain close. The systematic movement of large numbers of atoms led to some to refer to these as military transformations in contrast to civilian diffusion-based phase changes, initially by Frederick Charles Frank and John Wyrill Christian.

Austempering is heat treatment that is applied to ferrous metals, most notably steel and ductile iron. In steel it produces a bainite microstructure whereas in cast irons it produces a structure of acicular ferrite and high carbon, stabilized austenite known as ausferrite. It is primarily used to improve mechanical properties or reduce / eliminate distortion. Austempering is defined by both the process and the resultant microstructure. Typical austempering process parameters applied to an unsuitable material will not result in the formation of bainite or ausferrite and thus the final product will not be called austempered. Both microstructures may also be produced via other methods. For example, they may be produced as-cast or air cooled with the proper alloy content. These materials are also not referred to as austempered.
TRIP steel are a class of high-strength steel alloys typically used in naval and marine applications and in the automotive industry. TRIP stands for "Transformation induced plasticity," which implies a phase transformation in the material, typically when a stress is applied. These alloys are known to possess an outstanding combination of strength and ductility.
A nickel titanium rotary file is an engine-driven tapered and pointed endodontic instrument made of nickel titanium alloy with cutting edges used to mechanically shape and prepare the root canals during endodontic therapy or to remove the root canal obturating material while performing retreatment. The first nickel titanium rotary file was introduced to the market in 1991. Superelasticity and shape memory are the properties that make nickel titanium rotary files very flexible. The high flexibility makes them superior to stainless steel files for the purpose of rotary root canal preparation. The use of nickel titanium rotary files in dentistry is a common practice.












