Related Research Articles

In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy.

The neutron is a subatomic particle, symbol
n
or
n0
, which has no electric charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, they are both referred to as nucleons. Nucleons have a mass of approximately one atomic mass unit, or dalton. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks.

Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay.

In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (t1/2) for that collection, can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms has no known limits and spans a time range of over 55 orders of magnitude.
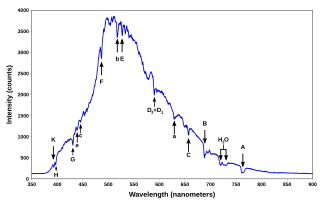
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible.
Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.

The effects of a nuclear explosion on its immediate vicinity are typically much more destructive and multifaceted than those caused by conventional explosives. In most cases, the energy released from a nuclear weapon detonated within the lower atmosphere can be approximately divided into four basic categories:

A mushroom cloud is a distinctive mushroom-shaped flammagenitus cloud of debris, smoke, and usually condensed water vapour resulting from a large explosion. The effect is most commonly associated with a nuclear explosion, but any sufficiently energetic detonation or deflagration will produce a similar effect. They can be caused by powerful conventional weapons, including thermobaric weapons such as the ATBIP and GBU-43/B MOAB. Some volcanic eruptions and impact events can produce natural mushroom clouds.

Neutron radiation is a form of ionizing radiation that presents as free neutrons. Typical phenomena are nuclear fission or nuclear fusion causing the release of free neutrons, which then react with nuclei of other atoms to form new nuclides—which, in turn, may trigger further neutron radiation. Free neutrons are unstable, decaying into a proton, an electron, plus an electron antineutrino. Free neutrons have a mean lifetime of 887 seconds.

Radioactive contamination, also called radiological pollution, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids, or gases, where their presence is unintended or undesirable.
Radiation hardening is the process of making electronic components and circuits resistant to damage or malfunction caused by high levels of ionizing radiation, especially for environments in outer space, around nuclear reactors and particle accelerators, or during nuclear accidents or nuclear warfare.

Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products, and neutrons. Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.

A nuclear explosion is an explosion that occurs as a result of the rapid release of energy from a high-speed nuclear reaction. The driving reaction may be nuclear fission or nuclear fusion or a multi-stage cascading combination of the two, though to date all fusion-based weapons have used a fission device to initiate fusion, and a pure fusion weapon remains a hypothetical device. Nuclear explosions are used in nuclear weapons and nuclear testing.
Radiation damage is the effect of ionizing radiation on physical objects including non-living structural materials. It can be either detrimental or beneficial for materials.
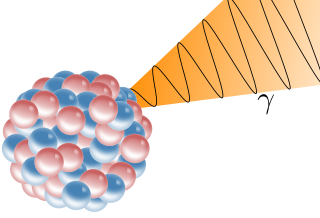
A gamma ray, also known as gamma radiation (symbol
γ
), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (3×1019 Hz) and wavelengths less than 10 picometers (1×10−11 m), gamma ray photons have the highest photon energy of any form of electromagnetic radiation. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; in 1900, he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power.

Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+ or 4
2He2+ indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom 4
2He.
Radiation materials science is a subfield of materials science which studies the interaction of radiation with matter: a broad subject covering many forms of irradiation and of matter.

Ion tracks are damage-trails created by swift heavy ions penetrating through solids, which may be sufficiently-contiguous for chemical etching in a variety of crystalline, glassy, and/or polymeric solids. They are associated with cylindrical damage-regions several nanometers in diameter and can be studied by Rutherford backscattering spectrometry (RBS), transmission electron microscopy (TEM), small-angle neutron scattering (SANS), small-angle X-ray scattering (SAXS) or gas permeation.
Travel outside the Earth's protective atmosphere, magnetosphere, and in free fall can harm human health, and understanding such harm is essential for successful crewed spaceflight. Potential effects on the central nervous system (CNS) are particularly important. A vigorous ground-based cellular and animal model research program will help quantify the risk to the CNS from space radiation exposure on future long distance space missions and promote the development of optimized countermeasures.
References
- ↑ Fernet, Marie; Hall, Janet (2020). "Radiation Sensitivity". In Schwab, Manfred (ed.). Encyclopedia of Cancer. Springer Science+Business Media. pp. 1–3. doi:10.1007/978-3-642-27841-9. ISBN 978-3-642-27841-9.
- ↑ "Individual Radiation Sensitivity". Bundesamt für Strahlenschutz . August 19, 2021. Retrieved January 23, 2023.
- ↑ Rajaraman, P.; Hauptmann, M.; Bouffler, S.; Wojcik, A. (April 12, 2018). "Human Individual Radiation Sensitivity and Prospects for Prediction". Annals of the ICRP. 47 (3–4). SAGE Publishing: 126–141. doi:10.1177/0146645318764091. ISSN 1872-969X. PMID 29648458. S2CID 4792165.