In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originated from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc, which have been dissolved into liquid solutions.

In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands; without mirroring one of them, hands cannot be superposed onto each other. No amount of reorientation in three spatial dimensions will allow the four unique groups on the chiral carbon to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers.

Vladimir Prelog was a Croatian-Swiss organic chemist who received the 1975 Nobel Prize in chemistry for his research into the stereochemistry of organic molecules and reactions. Prelog was born and grew up in Sarajevo. He lived and worked in Prague, Zagreb and Zürich during his lifetime.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."

Liquid chromatography–mass spectrometry (LC–MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry (MS). Coupled chromatography – MS systems are popular in chemical analysis because the individual capabilities of each technique are enhanced synergistically. While liquid chromatography separates mixtures with multiple components, mass spectrometry provides spectral information that may help to identify each separated component. MS is not only sensitive, but provides selective detection, relieving the need for complete chromatographic separation. LC–MS is also appropriate for metabolomics because of its good coverage of a wide range of chemicals. This tandem technique can be used to analyze biochemical, organic, and inorganic compounds commonly found in complex samples of environmental and biological origin. Therefore, LC–MS may be applied in a wide range of sectors including biotechnology, environment monitoring, food processing, and pharmaceutical, agrochemical, and cosmetic industries. Since the early 2000s, LC–MS has also begun to be used in clinical applications.

Biocatalysis refers to the use of living (biological) systems or their parts to speed up (catalyze) chemical reactions. In biocatalytic processes, natural catalysts, such as enzymes, perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task. Modern biotechnology, specifically directed evolution, has made the production of modified or non-natural enzymes possible. This has enabled the development of enzymes that can catalyze novel small molecule transformations that may be difficult or impossible using classical synthetic organic chemistry. Utilizing natural or modified enzymes to perform organic synthesis is termed chemoenzymatic synthesis; the reactions performed by the enzyme are classified as chemoenzymatic reactions.
Chiral column chromatography is a variant of column chromatography that is employed for the separation of chiral compounds, i.e. enantiomers, in mixtures such as racemates or related compounds. The chiral stationary phase (CSP) is made of a support, usually silica based, on which a chiral reagent or a macromolecule with numerous chiral centers is bonded or immobilized.
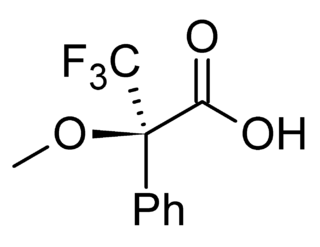
In analytical chemistry, A chiral derivatizing agent (CDA), also known as a chiral resolving reagent, is a derivatization reagent that is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present and determine the optical purity of a sample. Analysis can be conducted by spectroscopy or by chromatography. Some analytical techniques such as HPLC and NMR, in their most commons forms, cannot distinguish enantiomers within a sample, but can distinguish diastereomers. Therefore, converting a mixture of enantiomers to a corresponding mixture of diastereomers can allow analysis. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.
A monolithic HPLC column, or monolithic column, is a column used in high-performance liquid chromatography (HPLC). The internal structure of the monolithic column is created in such a way that many channels form inside the column. The material inside the column which separates the channels can be porous and functionalized. In contrast, most HPLC configurations use particulate packed columns; in these configurations, tiny beads of an inert substance, typically a modified silica, are used inside the column. Monolithic columns can be broken down into two categories, silica-based and polymer-based monoliths. Silica-based monoliths are known for their efficiency in separating smaller molecules while, polymer-based are known for separating large protein molecules.
An enantiopure drug is a pharmaceutical that is available in one specific enantiomeric form. Most biological molecules are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind differently to target receptors. Chirality can be observed when the geometric properties of an object is not superimposable with its mirror image. Two forms of a molecule are formed from a chiral carbon, these two forms are called enantiomers. One enantiomer of a drug may have a desired beneficial effect while the other may cause serious and undesired side effects, or sometimes even beneficial but entirely different effects. The desired enantiomer is known as an eutomer while the undesired enantiomer is known as the distomer. When equal amounts of both enantiomers are found in a mixture, the mixture is known as a racemic mixture. If a mixture for a drug does not have a 1:1 ratio of its enantiomers it is a candidate for an enantiopure drug. Advances in industrial chemical processes have made it economical for pharmaceutical manufacturers to take drugs that were originally marketed as a racemic mixture and market the individual enantiomers, either by specifically manufacturing the desired enantiomer or by resolving a racemic mixture. On a case-by-case basis, the U.S. Food and Drug Administration (FDA) has allowed single enantiomers of certain drugs to be marketed under a different name than the racemic mixture. Also case-by-case, the United States Patent Office has granted patents for single enantiomers of certain drugs. The regulatory review for marketing approval and for patenting is independent, and differs country by country.

Countercurrent chromatography is a form of liquid–liquid chromatography that uses a liquid stationary phase that is held in place by inertia of the molecules composing the stationary phase accelerating toward the center of a centrifuge due to centripetal force and is used to separate, identify, and quantify the chemical components of a mixture. In its broadest sense, countercurrent chromatography encompasses a collection of related liquid chromatography techniques that employ two immiscible liquid phases without a solid support. The two liquid phases come in contact with each other as at least one phase is pumped through a column, a hollow tube or a series of chambers connected with channels, which contains both phases. The resulting dynamic mixing and settling action allows the components to be separated by their respective solubilities in the two phases. A wide variety of two-phase solvent systems consisting of at least two immiscible liquids may be employed to provide the proper selectivity for the desired separation.
Amolak Chand Jain is an Indian natural product chemist, academic and the head of the department of chemistry at Delhi University, University of Jammu and Himachal Pradesh University. He is known for his studies on polyphenols, flavonoids and isoflavonoids and their syntheses. He is an elected fellow of the Indian National Science Academy and the National Academy of Sciences, India and a life member of the International Academy of Physical Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1969, for his contributions to chemical sciences.

Emanuel Gil-Av (Zimkin) was an Israeli chemist. The main emphasis of his work constituted chiral chromatography for the analytical separation of enantiomers.
Jessica R. Kramer is an American biomedical engineer working as an Assistant Professor of Bio-engineering and Adjunct Assistant Professor of Pharmaceutics and Pharmaceutical Chemistry at the University of Utah. Kramer’s research lab focuses on the synthesis and application of glycopolypeptides.
Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.

Chiral thin-layer chromatography is a variant of liquid chromatography that is employed for the separation of enantiomers. It is necessary to use either










