Related Research Articles
In genetics, an operon is a functioning unit of DNA containing a cluster of genes under the control of a single promoter. The genes are transcribed together into an mRNA strand and either translated together in the cytoplasm, or undergo splicing to create monocistronic mRNAs that are translated separately, i.e. several strands of mRNA that each encode a single gene product. The result of this is that the genes contained in the operon are either expressed together or not at all. Several genes must be co-transcribed to define an operon.
Pathogenicity islands (PAIs), as termed in 1990, are a distinct class of genomic islands acquired by microorganisms through horizontal gene transfer. Pathogenicity islands are found in both animal and plant pathogens. Additionally, PAIs are found in both gram-positive and gram-negative bacteria. They are transferred through horizontal gene transfer events such as transfer by a plasmid, phage, or conjugative transposon. Therefore, PAIs contribute to microorganisms' ability to evolve.

The trp operon is a group of genes that are transcribed together, encoding the enzymes that produce the amino acid tryptophan in bacteria. The trp operon was first characterized in Escherichia coli, and it has since been discovered in many other bacteria. The operon is regulated so that, when tryptophan is present in the environment, the genes for tryptophan synthesis are repressed.
The gene rpoS encodes the sigma factor sigma-38, a 37.8 kD protein in Escherichia coli. Sigma factors are proteins that regulate transcription in bacteria. Sigma factors can be activated in response to different environmental conditions. rpoS is transcribed in late exponential phase, and RpoS is the primary regulator of stationary phase genes. RpoS is a central regulator of the general stress response and operates in both a retroactive and a proactive manner: it not only allows the cell to survive environmental challenges, but it also prepares the cell for subsequent stresses (cross-protection). The transcriptional regulator CsgD is central to biofilm formation, controlling the expression of the curli structural and export proteins, and the diguanylate cyclase, adrA, which indirectly activates cellulose production. The rpoS gene most likely originated in the gammaproteobacteria.
The adaptive response is a form of direct DNA repair in E. coli that protects DNA from damage by external agents or by errors during replication. It is initiated against alkylation, particularly methylation, of guanine or thymine nucleotides or phosphate groups on the sugar-phosphate backbone of DNA. Under sustained exposure to low-level treatment with alkylating mutagens, E. coli can adapt to the presence of the mutagen, rendering subsequent treatment with high doses of the same agent less effective.

The micF RNA is a non-coding RNA stress response gene found in Escherichia coli and related bacteria that post-transcriptionally controls expression of the outer membrane porin gene ompF. The micF gene encodes a non-translated 93 nucleotide antisense RNA that binds its target ompF mRNA and regulates ompF expression by inhibiting translation and inducing degradation of the message. In addition, other factors, such as the RNA chaperone protein StpA also play a role in this regulatory system. The expression of micF is controlled by both environmental and internal stress factors. Four transcriptional regulators are known to bind the micF promoter region and activate micF expression.

The MicA RNA is a small non-coding RNA that was discovered in E. coli during a large scale screen. Expression of SraD is highly abundant in stationary phase, but low levels could be detected in exponentially growing cells as well.

The Hfq protein encoded by the hfq gene was discovered in 1968 as an Escherichia coli host factor that was essential for replication of the bacteriophage Qβ. It is now clear that Hfq is an abundant bacterial RNA binding protein which has many important physiological roles that are usually mediated by interacting with Hfq binding sRNA.

Leucine responsive protein, or Lrp, is a global regulator protein, meaning that it regulates the biosynthesis of leucine, as well as the other branched-chain amino acids, valine and isoleucine. In bacteria, it is encoded by the lrp gene.

Invasion gene associated RNA is a small non-coding RNA involved in regulating one of the major outer cell membrane porin proteins in Salmonella species.
VrrA is a non-coding RNA that is conserved across all Vibrio species of bacteria and acts as a repressor for the synthesis of the outer membrane protein OmpA. This non-coding RNA was initially identified from Tn5 transposon mutant libraries of Vibrio cholerae and its location within the bacterial genome was mapped to the intergenic region between genes VC1741 and VC1743 by RACE analysis.
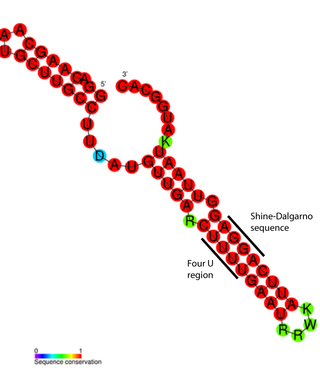
FourU thermometers are a class of non-coding RNA thermometers found in Salmonella. They are named 'FourU' due to the four highly conserved uridine nucleotides found directly opposite the Shine-Dalgarno sequence on hairpin II (pictured). RNA thermometers such as FourU control regulation of temperature via heat shock proteins in many prokaryotes. FourU thermometers are relatively small RNA molecules, only 57 nucleotides in length, and have a simple two-hairpin structure.
Bacterial small RNAs (bsRNA) are small RNAs produced by bacteria; they are 50- to 500-nucleotide non-coding RNA molecules, highly structured and containing several stem-loops. Numerous sRNAs have been identified using both computational analysis and laboratory-based techniques such as Northern blotting, microarrays and RNA-Seq in a number of bacterial species including Escherichia coli, the model pathogen Salmonella, the nitrogen-fixing alphaproteobacterium Sinorhizobium meliloti, marine cyanobacteria, Francisella tularensis, Streptococcus pyogenes, the pathogen Staphylococcus aureus, and the plant pathogen Xanthomonas oryzae pathovar oryzae. Bacterial sRNAs affect how genes are expressed within bacterial cells via interaction with mRNA or protein, and thus can affect a variety of bacterial functions like metabolism, virulence, environmental stress response, and structure.

An RNA thermometer is a temperature-sensitive non-coding RNA molecule which regulates gene expression. RNA thermometers often regulate genes required during either a heat shock or cold shock response, but have been implicated in other regulatory roles such as in pathogenicity and starvation.

In molecular biology, the ferric uptake regulator family is a family of bacterial proteins involved in regulating metal ion uptake and in metal homeostasis. The family is named for its founding member, known as the ferric uptake regulator or ferric uptake regulatory protein (Fur). Fur proteins are responsible for controlling the intracellular concentration of iron in many bacteria. Iron is essential for most organisms, but its concentration must be carefully managed over a wide range of environmental conditions; high concentrations can be toxic due to the formation of reactive oxygen species.
The bacterial stress response enables bacteria to survive adverse and fluctuating conditions in their immediate surroundings. Various bacterial mechanisms recognize different environmental changes and mount an appropriate response. A bacterial cell can react simultaneously to a wide variety of stresses and the various stress response systems interact with each other by a complex of global regulatory networks.
Oxidation response is stimulated by a disturbance in the balance between the production of reactive oxygen species and antioxidant responses, known as oxidative stress. Active species of oxygen naturally occur in aerobic cells and have both intracellular and extracellular sources. These species, if not controlled, damage all components of the cell, including proteins, lipids and DNA. Hence cells need to maintain a strong defense against the damage. The following table gives an idea of the antioxidant defense system in bacterial system.
The locus of enterocyte effacement-encoded regulator (Ler) is a regulatory protein that controls bacterial pathogenicity of enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic Escherichia coli (EHEC). More specifically, Ler regulates the locus of enterocyte effacement (LEE) pathogenicity island genes, which are responsible for creating intestinal attachment and effacing lesions and subsequent diarrhea: LEE1, LEE2, and LEE3. LEE1, 2, and 3 carry the information necessary for a type III secretion system. The transcript encoding the Ler protein is the open reading frame 1 on the LEE1 operon.

The Phosphate (Pho) regulon is a regulatory mechanism used for the conservation and management of inorganic phosphate within the cell. It was first discovered in Escherichia coli as an operating system for the bacterial strain, and was later identified in other species. The Pho system is composed of various components including extracellular enzymes and transporters that are capable of phosphate assimilation in addition to extracting inorganic phosphate from organic sources. This is an essential process since phosphate plays an important role in cellular membranes, genetic expression, and metabolism within the cell. Under low nutrient availability, the Pho regulon helps the cell survive and thrive despite a depletion of phosphate within the environment. When this occurs, phosphate starvation-inducible (psi) genes activate other proteins that aid in the transport of inorganic phosphate.
References
- 1 2 3 Lengeler, Joseph W.; Postma, P.W. (1999). "Global Regulatory Networks and Signal Transduction Pathways". In Lengeler, Joseph W.; Drews, Gerhart; Schlegel, Hans G. (eds.). Biology of the prokaryotes. Stuttgart: Thieme. pp. 498–9. ISBN 9781444313307.
- ↑ Regulon at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Nonaka, G; Blankschien, M; Herman, C; Gross, CA; Rhodius, VA (1 July 2006). "Regulon and promoter analysis of the E. coli heat-shock factor, sigma32, reveals a multifaceted cellular response to heat stress". Genes & Development. 20 (13): 1776–89. doi:10.1101/gad.1428206. PMC 1522074 . PMID 16818608.
- ↑ Lamarche, MG; Wanner, BL; Crépin, S; Harel, J (May 2008). "The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis". FEMS Microbiology Reviews. 32 (3): 461–73. doi: 10.1111/j.1574-6976.2008.00101.x . PMID 18248418.
- ↑ Hacker, J; Blum-Oehler, G; Mühldorfer, I; Tschäpe, H (March 1997). "Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution". Molecular Microbiology. 23 (6): 1089–97. doi: 10.1046/j.1365-2958.1997.3101672.x . PMID 9106201.
- ↑ Landini, P; Volkert, MR (December 2000). "Regulatory responses of the adaptive response to alkylation damage: a simple regulon with complex regulatory features". Journal of Bacteriology. 182 (23): 6543–9. doi:10.1128/jb.182.23.6543-6549.2000. PMC 111391 . PMID 11073893.
- ↑ Michael Hecker; Stefan Müllner (18 July 2003). Proteomics of Microorganisms: Fundamental Aspects and Application. Springer Science & Business Media. p. 66. ISBN 978-3-540-00546-9.
- ↑ Quinn, HJ; Cameron, AD; Dorman, CJ (March 2014). "Bacterial regulon evolution: distinct responses and roles for the identical OmpR proteins of Salmonella Typhimurium and Escherichia coli in the acid stress response". PLOS Genetics. 10 (3): e1004215. doi: 10.1371/journal.pgen.1004215 . PMC 3945435 . PMID 24603618.