Related Research Articles

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer and is classified as a Group 2A (probable) carcinogen.

CD36, also known as platelet glycoprotein 4, fatty acid translocase (FAT), scavenger receptor class B member 3 (SCARB3), and glycoproteins 88 (GP88), IIIb (GPIIIB), or IV (GPIV) is a protein that in humans is encoded by the CD36 gene. The CD36 antigen is an integral membrane protein found on the surface of many cell types in vertebrate animals. It imports fatty acids inside cells and is a member of the class B scavenger receptor family of cell surface proteins. CD36 binds many ligands including collagen, thrombospondin, erythrocytes parasitized with Plasmodium falciparum, oxidized low density lipoprotein, native lipoproteins, oxidized phospholipids, and long-chain fatty acids.

Merozoitesurface proteins are both integral and peripheral membrane proteins found on the surface of a merozoite, an early life cycle stage of a protozoan. Merozoite surface proteins, or MSPs, are important in understanding malaria, a disease caused by protozoans of the genus Plasmodium. During the asexual blood stage of its life cycle, the malaria parasite enters red blood cells to replicate itself, causing the classic symptoms of malaria. These surface protein complexes are involved in many interactions of the parasite with red blood cells and are therefore an important topic of study for scientists aiming to combat malaria.

Micronemes are secretory organelles, possessed by parasitic apicomplexans. Micronemes are located on the apical third of the protozoan body. They are surrounded by a typical unit membrane. On electron microscopy they have an electron-dense matrix due to the high protein content. They are specialized secretory organelles important for host-cell invasion and gliding motility.
A blocking antibody is an antibody that does not have a reaction when combined with an antigen, but prevents other antibodies from combining with that antigen. This function of blocking antibodies has had a variety of clinical and experimental uses.
Malaria prophylaxis is the preventive treatment of malaria. Several malaria vaccines are under development.
Malaria antigen detection tests are a group of commercially available rapid diagnostic tests of the rapid antigen test type that allow quick diagnosis of malaria by people who are not otherwise skilled in traditional laboratory techniques for diagnosing malaria or in situations where such equipment is not available. There are currently over 20 such tests commercially available. The first malaria antigen suitable as target for such a test was a soluble glycolytic enzyme Glutamate dehydrogenase. None of the rapid tests are currently as sensitive as a thick blood film, nor as cheap. A major drawback in the use of all current dipstick methods is that the result is essentially qualitative. In many endemic areas of tropical Africa, however, the quantitative assessment of parasitaemia is important, as a large percentage of the population will test positive in any qualitative assay.
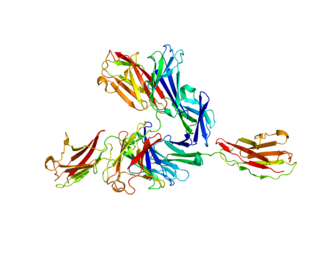
Basigin (BSG) also known as extracellular matrix metalloproteinase inducer (EMMPRIN) or cluster of differentiation 147 (CD147) is a protein that in humans is encoded by the BSG gene. This protein is a determinant for the Ok blood group system. There are three known antigens in the Ok system; the most common being Oka, OK2 and OK3. Basigin has been shown to be an essential receptor on red blood cells for the human malaria parasite, Plasmodium falciparum. The common isoform of basigin (basigin-2) has two immunoglobulin domains, and the extended form basigin-1 has three.
Malaria vaccines are vaccines that prevent malaria, a mosquito-borne infectious disease which annually affects an estimated 247 million people worldwide and causes 619,000 deaths. The first approved vaccine for malaria is RTS,S, known by the brand name Mosquirix. As of April 2023, the vaccine has been given to 1.5 million children living in areas with moderate-to-high malaria transmission. It requires at least three doses in infants by age 2, and a fourth dose extends the protection for another 1–2 years. The vaccine reduces hospital admissions from severe malaria by around 30%.
Human genetic resistance to malaria refers to inherited changes in the DNA of humans which increase resistance to malaria and result in increased survival of individuals with those genetic changes. The existence of these genotypes is likely due to evolutionary pressure exerted by parasites of the genus Plasmodium which cause malaria. Since malaria infects red blood cells, these genetic changes are most common alterations to molecules essential for red blood cell function, such as hemoglobin or other cellular proteins or enzymes of red blood cells. These alterations generally protect red blood cells from invasion by Plasmodium parasites or replication of parasites within the red blood cell.
Pregnancy-associated malaria (PAM) or placental malaria is a presentation of the common illness that is particularly life-threatening to both mother and developing fetus. PAM is caused primarily by infection with Plasmodium falciparum, the most dangerous of the four species of malaria-causing parasites that infect humans. During pregnancy, a woman faces a much higher risk of contracting malaria and of associated complications. Prevention and treatment of malaria are essential components of prenatal care in areas where the parasite is endemic – tropical and subtropical geographic areas. Placental malaria has also been demonstrated to occur in animal models, including in rodent and non-human primate models.

In molecular biology, apical membrane antigen 1 is a novel antigen of Plasmodium falciparum which has been cloned. It contains a hydrophobic domain typical of an integral membrane protein. The antigen is designated apical membrane antigen 1 (AMA-1) by virtue of appearing to be located in the apical complex. AMA-1 appears to be transported to the merozoite surface close to the time of schizont rupture.

In molecular biology, Duffy binding proteins are found in Plasmodium. Plasmodium vivax and Plasmodium knowlesi merozoites invade Homo sapiens erythrocytes that express Duffy blood group surface determinants. The Duffy receptor family is localised in micronemes, an organelle found in all organisms of the phylum Apicomplexa.
Russell J. Howard is an Australian-born executive, entrepreneur and scientist. He was a pioneer in the fields of molecular parasitology, especially malaria, and in leading the commercialisation of one of the most important methods used widely today in molecular biology today called “DNA shuffling" or "Molecular breeding", a form of "Directed evolution".
Circumsporozoite protein (CSP) is a secreted protein of the sporozoite stage of the malaria parasite and is the antigenic target of RTS,S and other malaria vaccines. The amino-acid sequence of CSP consists of an immunodominant central repeat region flanked by conserved motifs at the N- and C- termini that are implicated in protein processing as the parasite travels from the mosquito to the mammalian vector. The amino acid sequence of CSP was determined in 1984.
KAHRP is a protein expressed by Plasmodium falciparum infecting erythrocytes. KAHRP is a major component of knobs, feature found on Plasmodium falciparum infected erythrocytes.
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a family of proteins present on the membrane surface of red blood cells that are infected by the malarial parasite Plasmodium falciparum. PfEMP1 is synthesized during the parasite's blood stage inside the RBC, during which the clinical symptoms of falciparum malaria are manifested. Acting as both an antigen and adhesion protein, it is thought to play a key role in the high level of virulence associated with P. falciparum. It was discovered in 1984 when it was reported that infected RBCs had unusually large-sized cell membrane proteins, and these proteins had antibody-binding (antigenic) properties. An elusive protein, its chemical structure and molecular properties were revealed only after a decade, in 1995. It is now established that there is not one but a large family of PfEMP1 proteins, genetically regulated (encoded) by a group of about 60 genes called var. Each P. falciparum is able to switch on and off specific var genes to produce a functionally different protein, thereby evading the host's immune system. RBCs carrying PfEMP1 on their surface stick to endothelial cells, which facilitates further binding with uninfected RBCs, ultimately helping the parasite to both spread to other RBCs as well as bringing about the fatal symptoms of P. falciparum malaria.
Maurer's clefts are membranous structures seen in the red blood cell during infection with Plasmodium falciparum. The function and contents of Maurer's clefts are not completely known; however, they appear to play a role in trafficking of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) and other adhesins to the red blood cell surface.

The Plasmodium helical interspersed subtelomeric proteins (PHIST) or ring-infected erythrocyte surface antigens (RESA) are a family of protein domains found in the malaria-causing Plasmodium species. It was initially identified as a short four-helical conserved region in the single-domain export proteins, but the identification of this part associated with a DnaJ domain in P. falciparum RESA has led to its reclassification as the RESA N-terminal domain. This domain has been classified into three subfamilies, PHISTa, PHISTb, and PHISTc.
Wai-Hong Tham is a Malaysian professor at the University of Melbourne and the Walter and Eliza Hall Institute of Medical Research (WEHI), and joint head of the division of Infectious Disease and Immune Defense. She researches the molecular biology of the malaria parasite Plasmodium vivax.
References
- 1 2 Iyer J, Grüner AC, Rénia L, Snounou G, Preiser PR (July 2007). "Invasion of host cells by malaria parasites: a tale of two protein families". Molecular Microbiology. 65 (2): 231–49. doi: 10.1111/j.1365-2958.2007.05791.x . PMID 17630968.
- ↑ Lopaticki S, Maier AG, Thompson J, Wilson DW, Tham WH, Triglia T, et al. (March 2011). "Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites". Infection and Immunity. 79 (3): 1107–17. doi:10.1128/IAI.01021-10. PMC 3067488 . PMID 21149582.
- 1 2 Wright KE, Hjerrild KA, Bartlett J, Douglas AD, Jin J, Brown RE, et al. (November 2014). "Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies". Nature. 515 (7527): 427–30. Bibcode:2014Natur.515..427W. doi:10.1038/nature13715. PMC 4240730 . PMID 25132548.
- ↑ Wong W, Huang R, Menant S, Hong C, Sandow JJ, Birkinshaw RW, et al. (January 2019). "Structure of Plasmodium falciparum Rh5-CyRPA-Ripr invasion complex". Nature. 565 (7737): 118–121. doi:10.1038/s41586-018-0779-6. PMID 30542156. S2CID 54472333.
- ↑ Ragotte RJ, Higgins MK, Draper SJ (June 2020). "The RH5-CyRPA-Ripr Complex as a Malaria Vaccine Target". Trends in Parasitology. 36 (6): 545–559. doi:10.1016/j.pt.2020.04.003. PMC 7246332 . PMID 32359873.
- ↑ Cowman AF, Tonkin CJ, Tham WH, Duraisingh MT (August 2017). "The Molecular Basis of Erythrocyte Invasion by Malaria Parasites". Cell Host & Microbe. 22 (2): 232–245. doi: 10.1016/j.chom.2017.07.003 . PMID 28799908.
- ↑ Tham WH, Wilson DW, Lopaticki S, Schmidt CQ, Tetteh-Quarcoo PB, Barlow PN, et al. (October 2010). "Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand". Proceedings of the National Academy of Sciences of the United States of America. 107 (40): 17327–32. Bibcode:2010PNAS..10717327T. doi: 10.1073/pnas.1008151107 . PMC 2951459 . PMID 20855594.
- ↑ Grüber A, Gunalan K, Ramalingam JK, Manimekalai MS, Grüber G, Preiser PR (July 2011). "Structural characterization of the erythrocyte binding domain of the reticulocyte binding protein homologue family of Plasmodium yoelii". Infection and Immunity. 79 (7): 2880–8. doi:10.1128/IAI.01326-10. PMC 3191949 . PMID 21482683.