
Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.

The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the extracellular fluid of the central nervous system where neurons reside. The blood–brain barrier is formed by endothelial cells of the capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function.

A route of administration in pharmacology and toxicology is the way by which a drug, fluid, poison, or other substance is taken into the body.
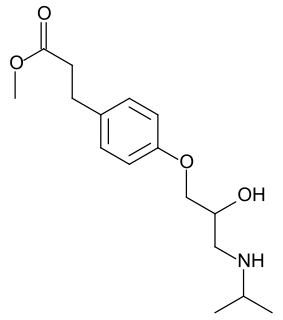
Esmolol, sold under the brand name Brevibloc, is a cardio selective beta1 receptor blocker with rapid onset, a very short duration of action, and no significant intrinsic sympathomimetic or membrane stabilising activity at therapeutic dosages.

Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.
Transcytosis is a type of transcellular transport in which various macromolecules are transported across the interior of a cell. Macromolecules are captured in vesicles on one side of the cell, drawn across the cell, and ejected on the other side. Examples of macromolecules transported include IgA, transferrin, and insulin. While transcytosis is most commonly observed in epithelial cells, the process is also present elsewhere. Blood capillaries are a well-known site for transcytosis, though it occurs in other cells, including neurons, osteoclasts and M cells of the intestine.

Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to drug preparation, route of administration, site-specific targeting, metabolism, and toxicity are used to optimize efficacy and safety, and to improve patient convenience and compliance. Drug delivery is aimed at altering a drug's pharmacokinetics and specificity by formulating it with different excipients, drug carriers, and medical devices. There is additional emphasis on increasing the bioavailability and duration of action of a drug to improve therapeutic outcomes. Some research has also been focused on improving safety for the person administering the medication. For example, several types of microneedle patches have been developed for administering vaccines and other medications to reduce the risk of needlestick injury.

Loteprednol is a topical corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax and Loterex.
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.
Therapeutic ultrasound refers generally to any type of ultrasonic procedure that uses ultrasound for therapeutic benefit. Physiotherapeutic ultrasound was introduced into clinical practice in the 1950s, with lithotripsy introduced in the 1980s. Others are at various stages in transitioning from research to clinical use: HIFU, targeted ultrasound drug delivery, trans-dermal ultrasound drug delivery, ultrasound hemostasis, cancer therapy, and ultrasound assisted thrombolysis It may use focused ultrasound (FUS) or unfocused ultrasound.

Transferrin receptor protein 1 (TfR1), also known as Cluster of Differentiation 71 (CD71), is a protein that in humans is encoded by the TFRC gene. TfR1 is required for iron import from transferrin into cells by endocytosis.
Microbubbles (MBs) are bubbles smaller than one hundredth of a millimetre in diameter, but larger than one micrometre. They have widespread application in industry, life science, and medicine. The composition of the bubble shell and filling material determine important design features such as buoyancy, crush strength, thermal conductivity, and acoustic properties.
A codrug or "mutual prodrug" consists of two synergistic drugs chemically linked together to a single molecule, in order to improve the drug delivery properties of one or both drugs.
Drug delivery to the brain is the process of passing therapeutically active molecules across the blood–brain barrier into the brain. This is a complex process that must take into account the complex anatomy of the brain as well as the restrictions imposed by the special junctions of the blood–brain barrier.

Diffuse midline glioma (DMG), previously called Diffuse Intrinsic Pontine Glioma (DIPG) is a fatal tumour that arises in the brainstem; most commonly in the pons or thalamus. DMG is believed to be caused by genetic mutations that cause epigenetic changes in cells of the developing nervous system, resulting in a failure of the cells to properly differentiate. Currently, the standard of care is fractionated external beam radiotherapy, as the tumour location precludes surgery, and chemotherapy has shown to be ineffective, however the estimated survival post-diagnosis remains only 9-15 months.
Nanoparticles for drug delivery to the brain is a method for transporting drug molecules across the blood–brain barrier (BBB) using nanoparticles. These drugs cross the BBB and deliver pharmaceuticals to the brain for therapeutic treatment of neurological disorders. These disorders include Parkinson's disease, Alzheimer's disease, schizophrenia, depression, and brain tumors. Part of the difficulty in finding cures for these central nervous system (CNS) disorders is that there is yet no truly efficient delivery method for drugs to cross the BBB. Antibiotics, antineoplastic agents, and a variety of CNS-active drugs, especially neuropeptides, are a few examples of molecules that cannot pass the BBB alone. With the aid of nanoparticle delivery systems, however, studies have shown that some drugs can now cross the BBB, and even exhibit lower toxicity and decrease adverse effects throughout the body. Toxicity is an important concept for pharmacology because high toxicity levels in the body could be detrimental to the patient by affecting other organs and disrupting their function. Further, the BBB is not the only physiological barrier for drug delivery to the brain. Other biological factors influence how drugs are transported throughout the body and how they target specific locations for action. Some of these pathophysiological factors include blood flow alterations, edema and increased intracranial pressure, metabolic perturbations, and altered gene expression and protein synthesis. Though there exist many obstacles that make developing a robust delivery system difficult, nanoparticles provide a promising mechanism for drug transport to the CNS.
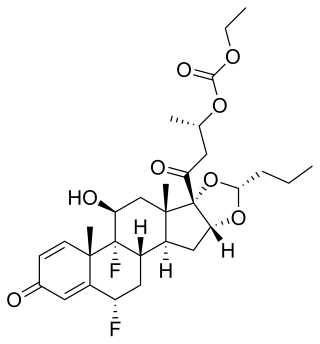
Itrocinonide is a synthetic glucocorticoid corticosteroid which was never marketed.

Sofpironium bromide is a drug used to treat hyperhidrosis. It was approved in Japan in 2020 as a 5% gel for the treament of primary axillary hyperhidrosis (PAH).

Focused ultrasound for intracrainial drug delivery is a non-invasive technique that uses high-frequency sound waves to disrupt tight junctions in the blood–brain barrier (BBB), allowing for increased passage of therapeutics into the brain. The BBB normally blocks nearly 98% of drugs from accessing the central nervous system, so FUS has the potential to address a major challenge in intracranial drug delivery by providing targeted and reversible BBB disruption. Using FUS to enhance drug delivery to the brain could significantly improve patient outcomes for a variety of diseases including Alzheimer's disease, Parkinson's disease, and brain cancer.
Convection-enhanced delivery (CED) is method of drug delivery in which drug is delivered into using bulk flow rather than conventional diffusion into the brain. This is done by utilizing catheters inserted into the target region of the brain and utilizing pressure to deliver the therapeutic to a target region. CED has been used to delivery drugs to the central nervous system (CNS) for diseases such as cancer, epilepsy, and Parkinson’s disease. CED has been used to deliver drugs to the CNS for its ability to bypass the blood–brain barrier (BBB) and target specific regions for targeted treatment, but current techniques using CED have failed to progress past clinical trials due to a variety of physical limitations associated with CED itself.












