Related Research Articles

Americium is a synthetic chemical element; it has symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element europium and was thus named after the Americas by analogy.

Beryllium is a chemical element; it has symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form minerals. Gemstones high in beryllium include beryl and chrysoberyl. It is a relatively rare element in the universe, usually occurring as a product of the spallation of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into heavier elements. Beryllium constitutes about 0.0004 percent by mass of Earth's crust. The world's annual beryllium production of 220 tons is usually manufactured by extraction from the mineral beryl, a difficult process because beryllium bonds strongly to oxygen.

Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay.

The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.

A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear power.

In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely more susceptible than fast neutrons to propagate a nuclear chain reaction of uranium-235 or other fissile isotope by colliding with their atomic nucleus.

The Atomic Age, also known as the Atomic Era, is the period of history following the detonation of the first nuclear weapon, The Gadget at the Trinity test in New Mexico, on 16 July 1945, during World War II. Although nuclear chain reactions had been hypothesized in 1933 and the first artificial self-sustaining nuclear chain reaction had taken place in December 1942, the Trinity test and the ensuing bombings of Hiroshima and Nagasaki that ended World War II represented the first large-scale use of nuclear technology and ushered in profound changes in sociopolitical thinking and the course of technological development.

Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
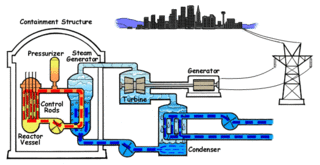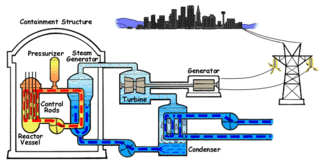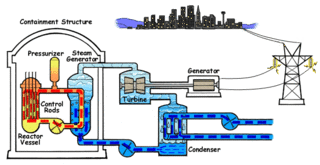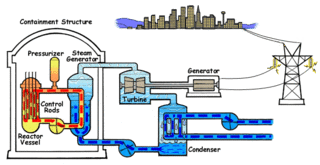
Nuclear propulsion includes a wide variety of propulsion methods that use some form of nuclear reaction as their primary power source. The idea of using nuclear material for propulsion dates back to the beginning of the 20th century. In 1903 it was hypothesized that radioactive material, radium, might be a suitable fuel for engines to propel cars, planes, and boats. H. G. Wells picked up this idea in his 1914 fiction work The World Set Free. Many aircraft carriers and submarines currently use uranium fueled nuclear reactors that can provide propulsion for long periods without refueling. There are also applications in the space sector with nuclear thermal and nuclear electric engines which could be more efficient than conventional rocket engines.
A criticality accident is an accidental uncontrolled nuclear fission chain reaction. It is sometimes referred to as a critical excursion, critical power excursion, or divergent chain reaction. Any such event involves the unintended accumulation or arrangement of a critical mass of fissile material, for example enriched uranium or plutonium. Criticality accidents can release potentially fatal radiation doses, if they occur in an unprotected environment.
David Charles Hahn, sometimes called the "Radioactive Boy Scout" or the "Nuclear Boy Scout", was an American nuclear radiation enthusiast who built a homemade neutron source at the age of seventeen.

Chicago Pile-1 (CP-1) was the world's first artificial nuclear reactor. On 2 December 1942, the first human-made self-sustaining nuclear chain reaction was initiated in CP-1 during an experiment led by Enrico Fermi. The secret development of the reactor was the first major technical achievement for the Manhattan Project, the Allied effort to create nuclear weapons during World War II. Developed by the Metallurgical Laboratory at the University of Chicago, CP-1 was built under the west viewing stands of the original Stagg Field. Although the project's civilian and military leaders had misgivings about the possibility of a disastrous runaway reaction, they trusted Fermi's safety calculations and decided they could carry out the experiment in a densely populated area. Fermi described the reactor as "a crude pile of black bricks and wooden timbers".
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95. Despite 243
Am being an order of magnitude longer lived than 241
Am, the former is harder to obtain than the latter as more of it is present in spent nuclear fuel.
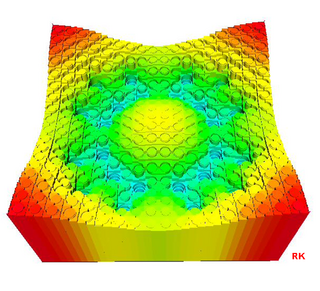
Nuclear reactor physics is the field of physics that studies and deals with the applied study and engineering applications of chain reaction to induce a controlled rate of fission in a nuclear reactor for the production of energy. Most nuclear reactors use a chain reaction to induce a controlled rate of nuclear fission in fissile material, releasing both energy and free neutrons. A reactor consists of an assembly of nuclear fuel, usually surrounded by a neutron moderator such as regular water, heavy water, graphite, or zirconium hydride, and fitted with mechanisms such as control rods which control the rate of the reaction.
The electricity sector in Sweden has three operational nuclear power plants with 6 operational nuclear reactors, which produce about 29.8% of the country's electricity. The nation's largest power station, Forsmark Nuclear Power Plant, has three reactors producing 3.3 GW and 14% of Sweden's electricity.
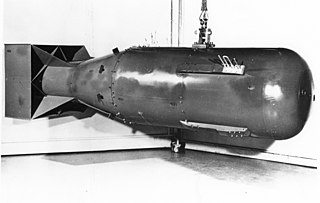
Weapons-grade nuclear material is any fissionable nuclear material that is pure enough to make a nuclear weapon or has properties that make it particularly suitable for nuclear weapons use. Plutonium and uranium in grades normally used in nuclear weapons are the most common examples.

Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+
or 4
2He2+
indicating a helium ion with a +2 charge. Once the ion gains electrons from its environment, the alpha particle becomes a normal helium atom 4
2He.
Startup neutron source is a neutron source used for stable and reliable initiation of nuclear chain reaction in nuclear reactors, when they are loaded with fresh nuclear fuel, whose neutron flux from spontaneous fission is insufficient for a reliable startup, or after prolonged shutdown periods. Neutron sources ensure a constant minimal population of neutrons in the reactor core, sufficient for a smooth startup. Without them, the reactor could suffer fast power excursions during startup from state with too few self-generated neutrons.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.

Americium-241 is an isotope of americium. Like all isotopes of americium, it is radioactive, with a half-life of 432.2 years. 241
Am
is the most common isotope of americium as well as the most prevalent isotope of americium in nuclear waste. It is commonly found in ionization type smoke detectors and is a potential fuel for long-lifetime radioisotope thermoelectric generators (RTGs). Its common parent nuclides are β− from 241
Pu
, EC from 241
Cm
, and α from 245
Bk
. 241
Am
is fissile and the critical mass of a bare sphere is 57.6–75.6 kilograms (127.0–166.7 lb) and a sphere diameter of 19–21 centimetres (7.5–8.3 in). Americium-241 has a specific activity of 3.43 Ci/g (126.91 GBq/g). It is commonly found in the form of americium-241 dioxide. This isotope also has one meta state, 241m
Am
, with an excitation energy of 2.2 MeV (0.35 pJ) and a half-life of 1.23 μs. The presence of americium-241 in plutonium is determined by the original concentration of plutonium-241 and the sample age. Because of the low penetration of alpha radiation, americium-241 only poses a health risk when ingested or inhaled. Older samples of plutonium containing 241
Pu
contain a buildup of 241
Am
. A chemical removal of americium-241 from reworked plutonium may be required in some cases.
References
- ↑ "Tänkte bygga kärnreaktor i köket" [I was thinking of building a nuclear reactor in the kitchen]. DN.SE (in Swedish).
- ↑ "Man tries to build nuke reactor in kitchen". Australian Broadcasting Corporation. ABC News. 5 August 2011.
- ↑ Nylander, Johan. "Swede tried to build nuke reactor in kitchen". swedishwire.com. Archived from the original on 1 December 2017. Retrieved 14 October 2022.
- see alternate source
- ↑ Ronson, Jon (30 October 2012). Lost at Sea. The Jon Ronson Mysteries. Penguin Group US. pp. 205 ff. ISBN 978-1-101-61242-2.
- ↑ "Swedish man arrested after trying to split atoms in his kitchen". The Daily Telegraph . London, UK. 3 August 2011.
- ↑ Ronson, Jon (3 February 2012). "DIY science: Should you try this at home?". The Guardian . London, UK.
- 1 2 "Swede admits home-made atom experiment was 'crazy'". London, UK: British Broadcasting Corporation. BBC News. 4 August 2011.
- ↑ "[no title cited]". Science News Daily. Archived from the original on 26 September 2018. Retrieved 14 October 2022.
- ↑ "Swedish man detained for building nuclear reactor in kitchen". The Australian .
- 1 2 "The weird story of the Swedish man who tried to build a nuclear reactor in his kitchen". Business Insider . 2 August 2011.
- ↑ Winter, Michael (August 2011). "Swedish man arrested over kitchen nuclear 'reactor'". USA Today .
- ↑ "Swede detained for building nuclear reactor in kitchen". Wired UK. 3 August 2011. Archived from the original on 13 May 2016. Retrieved 10 September 2017.
- ↑ Shepherd, Chuck (18 December 2012). News of the Weird: Ironies. Andrews McMeel Publishing. pp. 16 ff. ISBN 978-1-4494-3779-4.
- ↑ "Atom splitting in my kitchen was a hobby, man tells Swedish police". The Guardian . 3 August 2011.
- ↑ Wilson, Charles. "Nuclear reactor in kitchen". Hobbies from Hell. Abacus News. Archived from the original on 3 December 2017. Retrieved 14 October 2022.
- ↑ "Swede speaks out about kitchen nuclear reactor". The Local. 5 August 2011.
- ↑ "Court decision" . Retrieved 23 May 2015– via Google Docs.