
Hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome is an autosomal dominant genetic condition that is associated with a high risk of colon cancer as well as other cancers including endometrial cancer, ovary, stomach, small intestine, hepatobiliary tract, upper urinary tract, brain, and skin. The increased risk for these cancers is due to inherited mutations that impair DNA mismatch repair. It is a type of cancer syndrome. Because patients with Lynch syndrome can have polyps, the term HNPCC has fallen out of favor.

Cowden syndrome is an autosomal dominant inherited condition characterized by benign overgrowths called hamartomas as well as an increased lifetime risk of breast, thyroid, uterine, and other cancers. It is often underdiagnosed due to variability in disease presentation, but 99% of patients report mucocutaneous symptoms by age 20-29. Despite some considering it a primarily dermatologic condition, Cowden's syndrome is a multi-system disorder that also includes neurodevelopmental disorders such as macrocephaly.

Xenotropic murine leukemia virus–related virus (XMRV) is a retrovirus which was first described in 2006 as an apparently novel human pathogen found in tissue samples from men with prostate cancer. Initial reports erroneously linked the virus to prostate cancer and later to chronic fatigue syndrome (CFS), leading to considerable interest in the scientific and patient communities, investigation of XMRV as a potential cause of multiple medical conditions, and public-health concerns about the safety of the donated blood supply.

BRCA1 associated protein-1 is a deubiquitinating enzyme that in humans is encoded by the BAP1 gene. BAP1 encodes an 80.4 kDa nuclear-localizing protein with a ubiquitin carboxy-terminal hydrolase (UCH) domain that gives BAP1 its deubiquitinase activity. Recent studies have shown that BAP1 and its fruit fly homolog, Calypso, are members of the polycomb-group proteins (PcG) of highly conserved transcriptional repressors required for long-term silencing of genes that regulate cell fate determination, stem cell pluripotency, and other developmental processes.
TOX high mobility group box family member 3, also known as TOX3, is a human gene.
Alan Ashworth, FRS is a British molecular biologist, noted for his work on genes involved in cancer susceptibility. He is currently the President of the UCSF Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco, a multidisciplinary research and clinical care organisation that is one of the largest cancer centres in the Western United States. He was previously CEO of the Institute of Cancer Research (ICR) in London.
Sir Michael Rudolf Stratton, is a British clinical scientist and the third director of the Wellcome Trust Sanger Institute. He currently heads the Cancer Genome Project and is a leader of the International Cancer Genome Consortium.
Sir Bruce Anthony John Ponder FMedSci FRS is an English geneticist and cancer researcher. He is Emeritus Professor of Oncology at the University of Cambridge and former director of the Cancer Research UK Cambridge Institute.
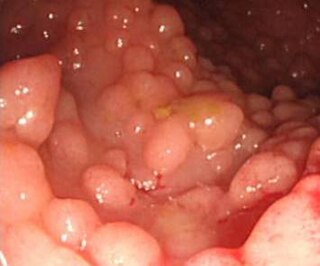
A cancer syndrome, or family cancer syndrome, is a genetic disorder in which inherited genetic mutations in one or more genes predispose the affected individuals to the development of cancers and may also cause the early onset of these cancers. Cancer syndromes often show not only a high lifetime risk of developing cancer, but also the development of multiple independent primary tumors.

Hereditary diffuse gastric cancer (HDGC) is an inherited genetic syndrome most often caused by an inactivating mutation in the E-cadherin gene (CDH1) located on chromosome 16. Individuals who inherit an inactive copy of the CDH1 gene are at significantly elevated risk for developing stomach cancer. For this reason, individuals with these mutations will often elect to under go prophylactic gastrectomy, or a complete removal of the stomach to prevent this cancer. Mutations in CDH1 are also associated with high risk of lobular breast cancers, and may be associated with a mildly elevated risk of colon cancer.
GT198 is a human oncogene located within the BRCA1 locus at chromosome 17q21. It encodes protein product named GT198, Hop2 or TBPIP. The GT198 gene is found to be mutated with its protein overexpressed in human cancers including breast and ovarian cancers.

Stephen Jacob Chanock (born April 15, 1956) is an American physician and geneticist. He currently serves as Director of the Division of Cancer Epidemiology and Genetics at the U.S. National Cancer Institute (NCI)1.
Charis Eng, M.D., Ph.D., is a Singapore-born physician-scientist and geneticist at the Cleveland Clinic, notable for identifying the PTEN gene. She is the Chairwoman and founding Director of the Genomic Medicine Institute of the Cleveland Clinic, founding Director and attending clinical cancer geneticist of the institute’s clinical component, the Center for Personalized Genetic Healthcare, and Professor and Vice Chairwoman of the Department of Genetics and Genome Sciences at Case Western Reserve University School of Medicine.

Nicola Jane Royle is a British geneticist who heads the Telomere Research Group in the Department of Genetics and Genome Biology at the University of Leicester. She is a specialist in the cellular processes that affect the stability of telomeres, the essential DNA-protein structures that cap the ends of chromosomes and play significant roles in cancer and ageing.
Suzanne Kathleen Chambers, is a Professor and Dean of the Faculty of Health at Sydney's University of Technology. She specialises in psycho-oncology, and has received Queen's Birthday honours. Chambers has worked on psycho-oncology, prostate cancer, health economics and psychological interventions including the distress and adjustments after cancer.

Jórunn Erla Eyfjörð is an Icelandic molecular biologist and professor emerita at the Faculty of Medicine of the University of Iceland. She is known for her research on breast cancer genetics.

Xiaohong Rose Yang is an American biomedical scientist researching the genetics of dysplastic nevus syndrome and chordoma, and etiologic heterogeneity of breast cancer. She is a senior investigator at the National Cancer Institute. Yang leads breast cancer studies in mainland China, Hong Kong, and Malaysia.
Hereditary lobular breast cancer is a rare inherited cancer predisposition associated with pathogenic CDH1 (gene) germline mutations, and without apparent correlation with the hereditary diffuse gastric cancer syndrome. Research studies identified novel CDH1 germline variants in women with diagnosed lobular breast cancer and without any family history of gastric carcinoma. Firstly, in 2018 Giovanni Corso et al. defined this syndrome as a new cancer predisposition and the Authors suggested additional clinical criteria to testing CDH1 in lobular breast cancer patients. In 2020, the International Gastric Cancer Linkage Consortium recognized officially that the hereditary lobular breast cancer is a novel and independent syndrome. To date, the are reported about 40 families clustering for lobular breast cancer and associated with CDH1 germline mutations but without association with diffuse gastric cancer.
Professor Patrick Francis Chinnery FRCP FRCPath FMedSci is a neurologist, clinician scientist, and Wellcome Trust Principal Research Fellow based in the Medical Research Council Mitochondrial Biology Unit and the University of Cambridge, where he is also Professor of Neurology and Head of the Department of Clinical Neurosciences.
Alexander (Sasha) Gusev is a computational biologist and an Assistant Professor of Medicine at Harvard Medical School.









