
In molecular biology, a riboswitch is a regulatory segment of a messenger RNA molecule that binds a small molecule, resulting in a change in production of the proteins encoded by the mRNA. Thus, an mRNA that contains a riboswitch is directly involved in regulating its own activity, in response to the concentrations of its effector molecule. The discovery that modern organisms use RNA to bind small molecules, and discriminate against closely related analogs, expanded the known natural capabilities of RNA beyond its ability to code for proteins, catalyze reactions, or to bind other RNA or protein macromolecules.

Cobalamin riboswitch is a cis-regulatory element which is widely distributed in 5' untranslated regions of vitamin B12 (Cobalamin) related genes in bacteria. Riboswitches are metabolite binding domains within certain messenger RNAs (mRNAs) that serve as precision sensors for their corresponding targets. Allosteric rearrangement of mRNA structure is mediated by ligand binding, and this results in modulation of gene expression or translation of mRNA to yield a protein. Cobalamin in the form of adenosylcobalamin (Ado-CBL) is known to repress expression of proteins for vitamin B12 biosynthesis via a post-transcriptional regulatory mechanism that involves direct binding of Ado-CBL to 5' UTRs in relevant genes, preventing ribosome binding and translation of those genes. Before proof of riboswitch function, a conserved sequence motif called the B12 box was identified that corresponds to a part of the cobalamin riboswitch, and a more complete conserved structure was identified. Variants of the riboswitch consensus have been identified.

The glucosamine-6-phosphate riboswitch ribozyme is an RNA structure that resides in the 5' untranslated region (UTR) of the mRNA transcript of the glmS gene. This RNA regulates the glmS gene by responding to concentrations of a specific metabolite, glucosamine-6-phosphate (GlcN6P), in addition to catalyzing a self-cleaving chemical reaction upon activation. This cleavage leads to the degradation of the mRNA that contains the ribozyme, and lowers production of GlcN6P. The glmS gene encodes for an enzyme glutamine-fructose-6-phosphate amidotransferase, which catalyzes the formation of GlcN6P, a compound essential for cell wall biosynthesis, from fructose-6-phosphate and glutamine. Thus, when GlcN6P levels are high, the glmS ribozyme is activated and the mRNA transcript is degraded but in the absence of GlcN6P the gene continues to be translated into glutamine-fructose-6-phosphate amidotransferase and GlcN6P is produced. GlcN6P is a cofactor for this cleavage reaction, as it directly participates as an acid-base catalyst. This RNA is the first riboswitch also found to be a self-cleaving ribozyme and, like many others, was discovered using a bioinformatics approach.
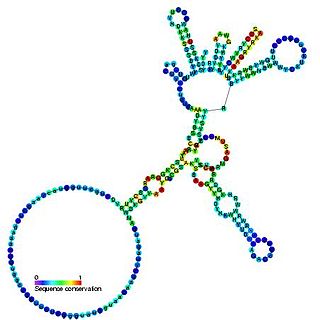
Group I introns are large self-splicing ribozymes. They catalyze their own excision from mRNA, tRNA and rRNA precursors in a wide range of organisms. The core secondary structure consists of nine paired regions (P1-P9). These fold to essentially two domains – the P4-P6 domain and the P3-P9 domain. The secondary structure mark-up for this family represents only this conserved core. Group I introns often have long open reading frames inserted in loop regions.

The Lysine riboswitch is a metabolite binding RNA element found within certain messenger RNAs that serve as a precision sensor for the amino acid lysine. Allosteric rearrangement of mRNA structure is mediated by ligand binding, and this results in modulation of gene expression. Lysine riboswitch are most abundant in Firmicutes and Gammaproteobacteria where they are found upstream of a number of genes involved in lysine biosynthesis, transport and catabolism. The lysine riboswitch has also been identified independently and called the L box.

The PreQ1-I riboswitch is a cis-acting element identified in bacteria which regulates expression of genes involved in biosynthesis of the nucleoside queuosine (Q) from GTP. PreQ1 (pre-queuosine1) is an intermediate in the queuosine pathway, and preQ1 riboswitch, as a type of riboswitch, is an RNA element that binds preQ1. The preQ1 riboswitch is distinguished by its unusually small aptamer, compared to other riboswitches. Its atomic-resolution three-dimensional structure has been determined, with the PDB ID 2L1V.

A purine riboswitch is a sequence of ribonucleotides in certain messenger RNA (mRNA) that selectively binds to purine ligands via a natural aptamer domain. This binding causes a conformational change in the mRNA that can affect translation by revealing an expression platform for a downstream gene, or by forming a translation-terminating stem-loop. The ultimate effects of such translational regulation often take action to manage an abundance of the instigating purine, and might produce proteins that facilitate purine metabolism or purine membrane uptake.

The SAM-II riboswitch is a RNA element found predominantly in alpha-proteobacteria that binds S-adenosyl methionine (SAM). Its structure and sequence appear to be unrelated to the SAM riboswitch found in Gram-positive bacteria. This SAM riboswitch is located upstream of the metA and metC genes in Agrobacterium tumefaciens, and other methionine and SAM biosynthesis genes in other alpha-proteobacteria. Like the other SAM riboswitch, it probably functions to turn off expression of these genes in response to elevated SAM levels. A significant variant of SAM-II riboswitches was found in Pelagibacter ubique and related marine bacteria and called SAM-V. Also, like many structured RNAs, SAM-II riboswitches can tolerate long loops between their stems.
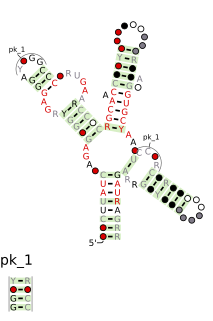
The SAM riboswitch is found upstream of a number of genes which code for proteins involved in methionine or cysteine biosynthesis in Gram-positive bacteria. Two SAM riboswitches in Bacillus subtilis that were experimentally studied act at the level of transcription termination control. The predicted secondary structure consists of a complex stem-loop region followed by a single stem-loop terminator region. An alternative and mutually exclusive form involves bases in the 3' segment of helix 1 with those in the 5' region of helix 5 to form a structure termed the anti-terminator form. When SAM is unbound, the anti-terminator sequence sequesters the terminator sequence so the terminator is unable to form, allowing the polymerase read-through the downstream gene. When S-Adenosyl methionine (SAM) is bound to the aptamer, the anti-terminator is sequestered by an anti-anti-terminator; the terminator forms and terminates the transcription. However, many SAM riboswitches are likely to regulate gene expression at the level of translation.

Usually found in gram-positive bacteria, the T box leader sequence is an RNA element that controls gene expression through the regulation of translation by binding directly to a specific tRNA and sensing its aminoacylation state. This interaction controls expression of downstream aminoacyl-tRNA synthetase genes, amino acid biosynthesis, and uptake-related genes in a negative feedback loop. The uncharged tRNA acts as the effector for transcription antitermination of genes in the T-box leader family. The anticodon of a specific tRNA base pairs to a specifier sequence within the T-box motif, and the NCCA acceptor tail of the tRNA base pairs to a conserved bulge in the T-box antiterminator hairpin.

The ykkC/yxkD leader is a conserved RNA structure found upstream of the ykkC and yxkD genes in Bacillus subtilis and related genes in other bacteria. The function of this family is unclear for many years although it has been suggested that it may function to switch on efflux pumps and detoxification systems in response to harmful environmental molecules. The Thermoanaerobacter tengcongensis sequence AE013027 overlaps with that of purine riboswitch suggesting that the two riboswitches may work in conjunction to regulate the upstream gene which codes for TTE0584 (Q8RC62), a member of the permease family.

Eukaryotic initiation factor 4A-III is a protein that in humans is encoded by the EIF4A3 gene.

SAH riboswitches are a kind of riboswitch that bind S-adenosylhomocysteine (SAH). When the coenzyme S-adenosylmethionine (SAM) is used in a methylation reaction, SAH is produced. SAH riboswitches typically up-regulate genes involved in recycling SAH to create more SAM. This is particularly relevant to cells, because high levels of SAH can be toxic. Originally identified by bioinformatics, SAH riboswitches are apparent in many species of bacteria, predominantly certain Proteobacteria and Actinobacteria. The atomic-resolution 3-dimensional structure of an SAH riboswitch has been solved using X-ray crystallography.

Cyclic di-GMP-I riboswitches are a class of riboswitch that specifically bind cyclic di-GMP, which is a second messenger that is used in a variety of microbial processes including virulence, motility and biofilm formation. Cyclic di-GMP-I riboswitches were originally identified by bioinformatics as a conserved RNA-like structure called the "GEMM motif". These riboswitches are present in a wide variety of bacteria, and are most common in Clostridia and certain varieties of Proteobacteria. The riboswitches are present in pathogens such as Clostridium difficile, Vibrio cholerae and Bacillus anthracis. Geobacter uraniumreducens is predicted to have 30 instances of this riboswitch in its genome. A bacteriophage that infects C. difficile is predicted to carry a cyclic di-GMP-I riboswitch, which it might use to detect and exploit the physiological state of bacteria that it infects.

The SAM–SAH riboswitch is a conserved RNA structure in certain bacteria that binds S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) and is therefore presumed to be a riboswitch. SAM–SAH riboswitches do not share any apparent structural resemblance to known riboswitches that bind SAM or SAH. The binding affinities for both compounds are similar, but binding for SAH is at least somewhat stronger. SAM–SAH riboswitches are exclusively found in Rhodobacterales, an order of alphaproteobacteria. They are always found in the apparent 5' untranslated regions of metK genes, which encode the enzyme that synthesizes SAM.

The pfl RNA motif refers to a conserved RNA structure present in some bacteria and originally discovered using bioinformatics. pfl RNAs are consistently present in genomic locations that likely correspond to the 5' untranslated regions of protein-coding genes. This arrangement in bacteria is commonly associated with cis-regulatory elements. Moreover, they are in presumed 5' UTRs of multiple non-homologous genes, suggesting that they function only in these locations. Additional evidence of cis-regulatory function came from the observation that predicted rho-independent transcription terminators overlap pfl RNAs. This overlap suggests that the alternate secondary structures of pfl RNA and the transcription terminator stem-loops compete with each other, and this is a common mechanism for cis gene control in bacteria.
SAM-V riboswitch is the fifth known riboswitch to bind S-adenosyl methionine (SAM). It was first discovered in the marine bacterium Candidatus Pelagibacter ubique and can also be found in marine metagenomes. SAM-V features a similar consensus sequence and secondary structure as the binding site of SAM-II riboswitch, but bioinformatics scans cluster the two aptamers independently. These similar binding pockets suggest that the two riboswitches have undergone convergent evolution.
High-throughput sequencing of RNA isolated by crosslinking immunoprecipitation is a genome-wide means of mapping protein–RNA binding sites or RNA modification sites in vivo. HITS-CLIP was originally used to generate genome-wide protein-RNA interaction maps for the neuron-specific RNA-binding protein and splicing factor NOVA1 and NOVA2; since then a number of other splicing factor maps have been generated, including those for PTB, RbFox2, SFRS1, hnRNP C, and even N6-Methyladenosine (m6A) mRNA modifications.
LysC is a prokaryotic aspartokinase involved in the biosynthesis of the amino acid lysine. It is found in a variety of bacteria, including Bacillus subtilis, Escherichia coli and Corynebacterium glutamicum. It is notable for containing a riboswitch, a structure in its messenger RNA that prevents its translation when bound to lysine. Such lysine riboswitch thus acts as a mechanism of negative feedback.
SAM-VI is a member of the riboswitch family. It is predominantly found in Bifidobacterium and exhibits some similarities to the SAM-III riboswitch class, but lacks most of the highly-conserved nucleotides of SAM-III class. SAM-VI aptamers bind the cofactor S-adenosylmethinine SAM and discriminate strongly against S-adenosylhomocysteine SAH. The class was discovered by further analysis of Bifido-meK motif RNAs.

















