In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.
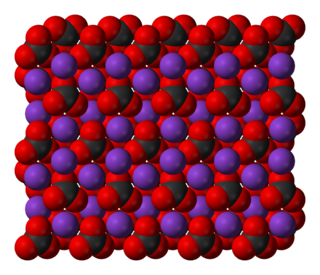
Potassium carbonate is the inorganic compound with the formula K2CO3. It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass.
The Leblanc process was an early industrial process for making soda ash used throughout the 19th century, named after its inventor, Nicolas Leblanc. It involved two stages: making sodium sulfate from sodium chloride, followed by reacting the sodium sulfate with coal and calcium carbonate to make sodium carbonate. The process gradually became obsolete after the development of the Solvay process.
The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate (soda ash, Na2CO3). The ammonia-soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. The ingredients for this are readily available and inexpensive: salt brine (from inland sources or from the sea) and limestone (from quarries). The worldwide production of soda ash in 2005 was estimated at 42 million tonnes, which is more than six kilograms (13 lb) per year for each person on Earth. Solvay-based chemical plants now produce roughly three-quarters of this supply, with the remaining being mined from natural deposits. This method superseded the Leblanc process.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.
ATC code A12Mineral supplements is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup A12 is part of the anatomical group A Alimentary tract and metabolism.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.

Kipp's apparatus, also called a Kipp generator, is an apparatus designed for preparation of small volumes of gases. It was invented around 1844 by the Dutch pharmacist Petrus Jacobus Kipp and widely used in chemical laboratories and for demonstrations in schools into the second half of the 20th century.

A chemical garden is a set of complex biological-looking structures created by mixing inorganic chemicals. Chemical gardening is an experiment in chemistry usually performed by adding metal salts, such as copper sulfate or cobalt(II) chloride, to an aqueous solution of sodium silicate. This results in the growth of plant-like forms in minutes to hours.

A chemistry set is an educational toy allowing the user to perform simple chemistry experiments.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
Calcium chlorate is the calcium salt of chloric acid, with the chemical formula Ca(ClO3)2. Like other chlorates, it is a strong oxidizer.

Chemische Fabrik Kalk (CFK) was a German chemicals company based in Kalk, a city district of Cologne. The company was founded in 1858 as Chemische Fabrik Vorster & Grüneberg, Cöln by Julius Vorster and Hermann Julius Grüneberg and was renamed to Chemische Fabrik Kalk GmbH in 1892. At times the company was the second-largest German producer of soda ash and was, with almost 2400 employees, one of the largest employers in Cologne. For decades the chimneys and the water tower of the factory dominated the skyline of Cologne-Kalk.
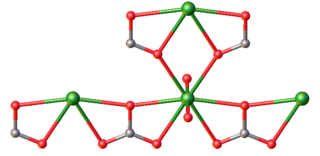
Uranyl carbonate refers to the inorganic compound with the formula UO2CO3. Also known by its mineral name rutherfordine, this material consists of uranyl (UO22+) and carbonate (CO32-). Like most uranyl salts, the compound is a polymeric, each uranium(VI) center being bonded to eight O atoms. Hydrolysis products of rutherfordine are also found in both the mineral and organic fractions of coal and its fly ash and is the main component of uranium in mine tailing seepage water.











