Related Research Articles

The human microbiome is the aggregate of all microbiota that reside on or within human tissues and biofluids along with the corresponding anatomical sites in which they reside, including the skin, mammary glands, seminal fluid, uterus, ovarian follicles, lung, saliva, oral mucosa, conjunctiva, biliary tract, and gastrointestinal tract. Types of human microbiota include bacteria, archaea, fungi, protists, and viruses. Though micro-animals can also live on the human body, they are typically excluded from this definition. In the context of genomics, the term human microbiome is sometimes used to refer to the collective genomes of resident microorganisms; however, the term human metagenome has the same meaning.

Gut microbiota, gut microbiome, or gut flora, are the microorganisms, including bacteria, archaea, fungi, and viruses, that live in the digestive tracts of animals. The gastrointestinal metagenome is the aggregate of all the genomes of the gut microbiota. The gut is the main location of the human microbiome. The gut microbiota has broad impacts, including effects on colonization, resistance to pathogens, maintaining the intestinal epithelium, metabolizing dietary and pharmaceutical compounds, controlling immune function, and even behavior through the gut–brain axis.

Bacteroides is a genus of Gram-negative, obligate anaerobic bacteria. Bacteroides species are non endospore-forming bacilli, and may be either motile or nonmotile, depending on the species. The DNA base composition is 40–48% GC. Unusual in bacterial organisms, Bacteroides membranes contain sphingolipids. They also contain meso-diaminopimelic acid in their peptidoglycan layer.

Bacteroides fragilis is an anaerobic, Gram-negative, pleomorphic to rod-shaped bacterium. It is part of the normal microbiota of the human colon and is generally commensal, but can cause infection if displaced into the bloodstream or surrounding tissue following surgery, disease, or trauma.
Dysbiosis is characterized by a disruption to the microbiome resulting in an imbalance in the microbiota, changes in their functional composition and metabolic activities, or a shift in their local distribution. For example, a part of the human microbiota such as the skin flora, gut flora, or vaginal flora, can become deranged, with normally dominating species underrepresented and normally outcompeted or contained species increasing to fill the void. Dysbiosis is most commonly reported as a condition in the gastrointestinal tract.
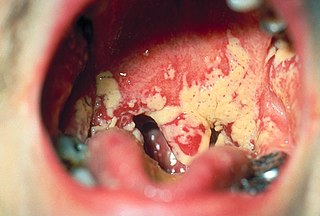
Oral microbiology is the study of the microorganisms (microbiota) of the oral cavity and their interactions between oral microorganisms or with the host. The environment present in the human mouth is suited to the growth of characteristic microorganisms found there. It provides a source of water and nutrients, as well as a moderate temperature. Resident microbes of the mouth adhere to the teeth and gums to resist mechanical flushing from the mouth to stomach where acid-sensitive microbes are destroyed by hydrochloric acid.

Long-term close-knit interactions between symbiotic microbes and their host can alter host immune system responses to other microorganisms, including pathogens, and are required to maintain proper homeostasis. The immune system is a host defense system consisting of anatomical physical barriers as well as physiological and cellular responses, which protect the host against harmful microorganisms while limiting host responses to harmless symbionts. Humans are home to 1013 to 1014 bacteria, roughly equivalent to the number of human cells, and while these bacteria can be pathogenic to their host most of them are mutually beneficial to both the host and bacteria.

Microbiota are the range of microorganisms that may be commensal, mutualistic, or pathogenic found in and on all multicellular organisms, including plants. Microbiota include bacteria, archaea, protists, fungi, and viruses, and have been found to be crucial for immunologic, hormonal, and metabolic homeostasis of their host.

The gut–brain axis is the two-way biochemical signaling that takes place between the gastrointestinal tract and the central nervous system (CNS). The "microbiota–gut–brain axis" includes the role of gut microbiota in the biochemical signaling events that take place between the GI tract and the CNS. Broadly defined, the gut–brain axis includes the central nervous system, neuroendocrine system, neuroimmune systems, the hypothalamic–pituitary–adrenal axis, sympathetic and parasympathetic arms of the autonomic nervous system, the enteric nervous system, vagus nerve, and the gut microbiota.
The altered Schaedler flora (ASF) is a community of eight bacterial species: two lactobacilli, one Bacteroides, one spiral bacterium of the Flexistipes genus, and four extremely oxygen sensitive (EOS) fusiform-shaped species. The bacteria are selected for their dominance and persistence in the normal microflora of mice, and for their ability to be isolated and grown in laboratory settings. Germ-free animals, mainly mice, are colonized with ASF for the purpose of studying the gastrointestinal (GI) tract. Intestinal mutualistic bacteria play an important role in affecting gene expression of the GI tract, immune responses, nutrient absorption, and pathogen resistance. The standardized microbial cocktail enabled the controlled study of microbe and host interactions, role of microbes, pathogen effects, and intestinal immunity and disease association, such as cancer, inflammatory bowel disease, diabetes, and other inflammatory or autoimmune diseases. Also, compared to germfree animals, ASF mice have fully developed immune system, resistance to opportunistic pathogens, and normal GI function and health, and are a great representation of normal mice.
Microbiota-accessible carbohydrates (MACs) are carbohydrates that are resistant to digestion by a host's metabolism, and are made available for gut microbes, as prebiotics, to ferment or metabolize into beneficial compounds, such as short chain fatty acids. The term, ‘‘microbiota-accessible carbohydrate’’ contributes to a conceptual framework for investigating and discussing the amount of metabolic activity that a specific food or carbohydrate can contribute to a host's microbiota.

A microbiome is the community of microorganisms that can usually be found living together in any given habitat. It was defined more precisely in 1988 by Whipps et al. as "a characteristic microbial community occupying a reasonably well-defined habitat which has distinct physio-chemical properties. The term thus not only refers to the microorganisms involved but also encompasses their theatre of activity". In 2020, an international panel of experts published the outcome of their discussions on the definition of the microbiome. They proposed a definition of the microbiome based on a revival of the "compact, clear, and comprehensive description of the term" as originally provided by Whipps et al., but supplemented with two explanatory paragraphs. The first explanatory paragraph pronounces the dynamic character of the microbiome, and the second explanatory paragraph clearly separates the term microbiota from the term microbiome.
The microbiota are the sum of all symbiotic microorganisms living on or in an organism. The fruit fly Drosophila melanogaster is a model organism and known as one of the most investigated organisms worldwide. The microbiota in flies is less complex than that found in humans. It still has an influence on the fitness of the fly, and it affects different life-history characteristics such as lifespan, resistance against pathogens (immunity) and metabolic processes (digestion). Considering the comprehensive toolkit available for research in Drosophila, analysis of its microbiome could enhance our understanding of similar processes in other types of host-microbiota interactions, including those involving humans. Microbiota plays key roles in the intestinal immune and metabolic responses via their fermentation product, acetate.
B. Brett Finlay, is a Canadian microbiologist well known for his contributions to understanding how microbes cause disease in people and developing new tools for fighting infections, as well as the role the microbiota plays in human health and disease. Science.ca describes him as one of the world's foremost experts on the molecular understanding of the ways bacteria infect their hosts. He also led the SARS Accelerated Vaccine Initiative (SAVI) and developed vaccines to SARS and a bovine vaccine to E. coli O157:H7. His current research interests focus on pathogenic E. coli and Salmonella pathogenicity, and the role of the microbiota in infections, asthma, and malnutrition. He is currently the UBC Peter Wall Distinguished Professor and a Professor in the Michael Smith Laboratories, Microbiology and Immunology, and Biochemistry and Molecular Biology, and Co-director and Senior Fellow for the CIFAR Humans and Microbes program. He is also co-author of the book Let Them Eat Dirt: Saving Your Child from an Oversanitized World and The Whole-Body Microbiome: How to Harness Microbes - Inside and Out - For Lifelong Health. Finlay is the author of over 500 publications in peer-reviewed journals and served as editor of several professional publications for many years.
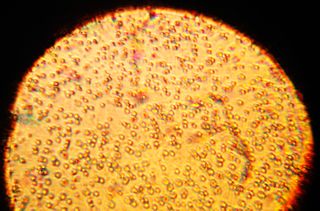
The human milk microbiota, also known as human milk probiotics (HMP), refers to the microbiota (community of microorganisms) residing in the human mammary glands and breast milk. Human breast milk has been traditionally assumed to be sterile, but more recently both microbial culture and culture-independent techniques have confirmed that human milk contains diverse communities of bacteria which are distinct from other microbial communities inhabiting the human body.

Elaine Yih-Nien Hsiao is an American biologist who is Professor in Biological Sciences at University of California, Los Angeles. Her research considers the microbes that impact human health. She was a 2022 Laureate for the Blavatnik Awards for Young Scientists.

Seed Health, popularly known as Seed, is an American health and life science company, most known for developing probiotics. Founded in 2015 by Ara Katz and Raja Dhir, Seed was founded to "use bacteria to improve human and environmental health". The company's main product, the probiotic DS-01, is sold direct to consumer, forgoing traditional brick-and-mortar retail.
Dennis Kasper is an American microbiologist and immunologist, and the William Ellery Channing Professor of Medicine and Professor of Immunology at Harvard Medical School. He leads the Kasper Laboratory within the Blavatnik Institute in the Department of Immunology at Harvard Medical School. He was also executive dean for academic programs at Harvard Medical School and director of the Channing Laboratory Department of Medicine at Brigham and Women's Hospital.
Bile salt hydrolases (BSH) are microbial enzymes that deconjugate primary bile acids. They catalyze the first step of bile acid metabolism and maintain the bile acid pool for further modification by the microbiota. BSH enzymes play a role in a range of host and microbe functions including host physiology, immunity, and protection from pathogens.

Phocaeicola vulgatus,, is a mutualistic anaerobic Gram negative rod bacteria commonly found in the human gut microbiome and isolated from feces. P. vulgatus has medical relevance and has been notable in scientific research due to its production of fatty acids, potential use as a probiotic, and associations with protecting against and worsening some inflammatory diseases. Due to the difficulties in culturing anaerobic bacteria, P. vulgatus is still highly uncharacterised so efforts are being made to make use of multi-omic approaches to investigate the human gut microbiome more thoroughly in hopes to fully understand the role of this species in the development of and protection against diseases, as well as its potential uses in medicine and research. Generally, P. vulgatus is considered as a beneficial bacteria that contributes to digestion and a balanced microbiome, but it has been known to cause opportunistic infections and induce or worsen inflammatory responses. Due to its abundance in the microbiome, some researchers are investigating these species in hopes that it will be a suitable model organism for gut microbiome research, like Bacteroides thetaiotaomicron.
References
- ↑ Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL (2005). "An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system". Cell. 122 (1): 107–18. doi: 10.1016/j.cell.2005.05.007 . PMID 16009137. S2CID 15708031.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Adams, Susan. "Drugs From Bugs: Why Gates, Zuck And Benioff Think The Next Blockbusters Will Come From Inside Your Gut". Forbes. Retrieved 2022-09-27.
In 2016, David Donabedian, a chemistry Ph.D. who was then a partner at Longwood Fund, a Boston venture capital firm, volunteered to raise the money and research power to move Mazmanian's biotech venture forward. The company, Waltham, Massachusetts–based Axial Biotherapeutics, has $55 million in backing and 30 employees.
- ↑ Quaglia, Sofia. "How your microbiome can improve your health". www.bbc.com. Retrieved 2022-09-27.
One company, Axial Therapeutics, wants to use microbiota analysis to better understand the relationship between microbes and the central nervous system in the hope of creating new pharmaceuticals.
- ↑ Lee, Yeji Jesse. "Meet the 10 neuroscience startups that have raised millions to treat mental health and cure neurologic diseases". Business Insider. Retrieved 2022-09-27.
Axial – $102.1 million
- ↑ Martino, Cameron; Zaramela, Livia S.; Gao, Bei; Embree, Mallory; Tarasova, Janna; Parker, Seth J.; Wang, Yanhan; Chu, Huikuan; Chen, Peng; Lee, Kuei-Chuan; Galzerani, Daniela Domingos; Gengatharan, Jivani M.; Lekbua, Asama; Neal, Maxwell; Knight, Rob (2022-08-08). "Acetate reprograms gut microbiota during alcohol consumption". Nature Communications. 13 (1): 4630. Bibcode:2022NatCo..13.4630M. doi:10.1038/s41467-022-31973-2. ISSN 2041-1723. PMC 9359997 . PMID 35941112.
- ↑ Smith, Peter Andrey (2015-06-23). "Can the Bacteria in Your Gut Explain Your Mood?". The New York Times. ISSN 0362-4331 . Retrieved 2022-09-27.
Mazmanian knew the results offered only a provisional explanation for why restrictive diets and antibacterial treatments seemed to help some children with autism: Altering the microbial composition might be changing the permeability of the intestine. The larger concept is, and this is pure speculation: Is a disease like autism really a disease of the brain or maybe a disease of the gut or some other aspect of physiology? Mazmanian said. For any disease in which such a link could be proved, he saw a future in drugs derived from these small molecules found inside microbes. (A company he co-founded, Symbiotix Biotherapies, is developing a complex sugar called PSA, which is associated with Bacteroides fragilis, into treatments for intestinal disease and multiple sclerosis.) In his view, the prescriptive solutions probably involve more than increasing our exposure to environmental microbes in soil, dogs or even fermented foods; he believed there were wholesale failures in the way we shared our microbes and inoculated children with these bacteria. So far, though, the only conclusion he could draw was that disorders once thought to be conditions of the brain might be symptoms of microbial disruptions, and it was the careful defining of these disruptions that promised to be helpful in the coming decades.
- ↑ "Autism's Gut Connection: Microbes Could Soon Lead to New Treatments". Discover Magazine. Retrieved 2022-09-27.
At 47, Mazmanian — with his shaved head, flannel shirt and skinny jeans — resembles a young, urban hipster on his way to write at the local café. Originally, literary life was his plan. Born in Lebanon to two Armenian refugees, neither of whom had more than a first-grade education, Mazmanian landed in the class of an energetic high school English teacher in California's San Fernando Valley, where his family first settled. The teacher recognized his gift for language and encouraged him to pursue a career in literature. Mazmanian enrolled at UCLA in 1990, planning to major in English.
- ↑ "uBiome Appoints Dr. Sarkis K. Mazmanian, PhD, MacArthur Genius and Luis & Nelly Soux Professor of Microbiology at Caltech, to its Scientific Advisory Board". markets.businessinsider.com. Retrieved 2022-09-27.
Soon after, he moved to Caltech and established his laboratory, which studies the beneficial bacterial molecules from the human gut microbiome as novel therapies for immunologic and neurologic disorders.
- ↑ "uBiome Appoints Dr. Sarkis K. Mazmanian, PhD, MacArthur Genius and Luis & Nelly Soux Professor of Microbiology at Caltech, to its Scientific Advisory Board". PRWeb. Retrieved 2022-09-27.