
The Shilov system is a classic example of catalytic C-H bond activation and oxidation which preferentially activates stronger C-H bonds over weaker C-H bonds for an overall partial oxidation. [1] [2] [3] [4] [5] [6] [7] [8]

The Shilov system is a classic example of catalytic C-H bond activation and oxidation which preferentially activates stronger C-H bonds over weaker C-H bonds for an overall partial oxidation. [1] [2] [3] [4] [5] [6] [7] [8]
The Shilov system was discovered by Alexander E. Shilov in 1969-1972 while investigating H/D exchange between isotopologues of CH4 and H2O catalyzed simple transition metal coordination complexes. The Shilov cycle is the partial oxidation of a hydrocarbon to an alcohol or alcohol precursor (RCl) catalyzed by PtIICl2 in an aqueous solution with [PtIVCl6]2− acting as the ultimate oxidant. The cycle consists of three major steps, the electrophilic activation of the C-H bond, oxidation of the complex, and the nucleophilic oxidation of the alkane substrate. An equivalent transformation is performed industrially by steam reforming methane to syngas then reducing the carbon monoxide to methanol. The transformation can also performed biologically by methane monooxygenase.
Overall Transformation
RH + H2O + [PtCl6]2− → ROH + 2H+ + PtCl2 + 4Cl−
The initial and rate limiting step involving the electrophilic activation of RH2C-H by a PtII center to produce a PtII-CH2R species and a proton. The mechanism of this activation is debated. One possibility is the oxidative addition of a sigma coordinated C-H bond followed by the reductive removal of the proton. Another is a sigma-bond metathesis involving the formation of the M-C bond and a H-Cl or H-O bond. Regardless it is this step that kinetically imparts the chemoselectivity to the overall transformation. Stronger, more electron-rich bonds are activated preferentially over weaker, more electron-poor bonds of species that have already been partially oxidized. This avoids a problem that plagues many partial oxidation processes, namely, the over-oxidation of substrate to thermodynamic sinks such as H2O and CO2.
In the next step the PtII-CH2R complex is oxidized by [PtIVCl6]2− to a PtIV-CH2R complex. There have been multiple studies to find a replacement oxidant that is less expensive than [PtIVCl6]2− or a method to regenerate [PtIVCl6]2−. It would be most advantageous to develop an electron train which would use oxygen as the ultimate oxidant. It is important that the oxidant preferentially oxidizes the PtII-CH2R species over the initial PtII species since PtIV complexes will not electrophilically activate a C-H bond of the alkane (although PtIV complexes electrophilically substitute hydrogens in aromatics - see refs. [1] and [2] ). Such premature oxidation shuts down the catalysis.
Finally the PtIV-CH2R undergoes nucleophilic attack by OH− or Cl− with the departure of PtII complex to regenerate the catalyst.

In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers. In the fossil fuel industries, hydrocarbon refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are carbon dioxide from the burning of fossil fuels, and methane released from natural gas handling and from agriculture.

Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. The term "unsaturated" means more hydrogen atoms may be added to the hydrocarbon to make it saturated. The configuration of an unsaturated carbons include straight chain, such as alkenes and alkynes, as well as branched chains and aromatic compounds.
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (F2, Cl2, Br2, I2). Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination.
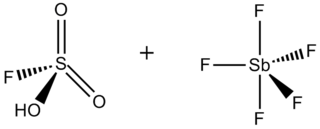
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted–Lewis superacid system was developed in the 1960s by the George Olah lab at Case Western Reserve University, and has been used to stabilize carbocations and hypercoordinated carbonium ions in liquid media. Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen.
The nitrosonium ion is NO+, in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: NOClO4, NOSO4H (nitrosylsulfuric acid, more descriptively written ONSO3OH) and NOBF4. The ClO−4 and BF−4 salts are slightly soluble in acetonitrile CH3CN. NOBF4 can be purified by sublimation at 200–250 °C and 0.01 mmHg (1.3 Pa).
Carbon–hydrogen bond functionalization is a type of reaction in which a carbon–hydrogen bond is cleaved and replaced with a carbon–X bond. The term usually implies that a transition metal is involved in the C-H cleavage process. Reactions classified by the term typically involve the hydrocarbon first to react with a metal catalyst to create an organometallic complex in which the hydrocarbon is coordinated to the inner-sphere of a metal, either via an intermediate "alkane or arene complex" or as a transition state leading to a "M−C" intermediate. The intermediate of this first step can then undergo subsequent reactions to produce the functionalized product. Important to this definition is the requirement that during the C–H cleavage event, the hydrocarbyl species remains associated in the inner-sphere and under the influence of "M".
In organometallic chemistry, agostic interaction refers to the interaction of a coordinatively-unsaturated transition metal with a C−H bond, when the two electrons involved in the C−H bond enter the empty d-orbital of the transition metal, resulting in a three-center two-electron bond. Many catalytic transformations, e.g. oxidative addition and reductive elimination, are proposed to proceed via intermediates featuring agostic interactions. Agostic interactions are observed throughout organometallic chemistry in alkyl, alkylidene, and polyenyl ligands.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

Alexander E. Shilov was a Russian chemist.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.
Oxidation with dioxiranes refers to the introduction of oxygen into organic molecules through the action of a dioxirane. Dioxiranes are well known for their oxidation of alkenes to epoxides; however, they are also able to oxidize other unsaturated functionality, heteroatoms, and alkane C-H bonds.
Georgiy Borisovich Shul’pin was born in 1946 in Moscow, Russia. He graduated with a M.S. degree in chemistry from the Chemistry Department of Moscow State University in 1969. Between 1969 and 1972, he was a postgraduate student at the Nesmeyanov Institute of Organoelement Compounds under the direction of Prof. A. N. Nesmeyanov and received his Ph.D. in organometallic chemistry in 1975. He received his Dr. of Sciences degree in 2013.
A transition metal oxo complex is a coordination complex containing an oxo ligand. Formally O2-, an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) as bridging ligands (Fig. 1). Oxo ligands stabilize high oxidation states of a metal. They are also found in several metalloproteins, for example in molybdenum cofactors and in many iron-containing enzymes. One of the earliest synthetic compounds to incorporate an oxo ligand is potassium ferrate (K2FeO4), which was likely prepared by Georg E. Stahl in 1702.
Roy A. Periana is a Guyanese-American organometallic chemist.
The White–Chen catalyst is an Iron-based coordination complex named after Professor M. Christina White and her graduate student Mark S. Chen. The catalyst is used along with hydrogen peroxide and acetic acid additive to oxidize aliphatic sp3 C-H bonds in organic synthesis. The catalyst is the first to allow for preparative and predictable aliphatic C–H oxidations over a broad range of organic substrates. Oxidations with the catalyst have proven to be remarkably predictable based on sterics, electronics, and stereoelectronics allowing for aliphatic C–H bonds to be thought of as a functional group in the streamlining of organic synthesis.
Methane functionalization is the process of converting methane in its gaseous state to another molecule with a functional group, typically methanol or acetic acid, through the use of transition metal catalysts.
Karen Ila Goldberg is an American chemist, currently the Vagelos Professor of Energy Research at University of Pennsylvania. Goldberg is most known for her work in inorganic and organometallic chemistry. Her most recent research focuses on catalysis, particularly on developing catalysts for oxidation, as well as the synthesis and activation of molecular oxygen. In 2018, Goldberg was elected to the National Academy of Sciences.