
A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: within the next 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.

The vanadium redox battery (VRB), also known as the vanadium flow battery (VFB) or vanadium redox flow battery (VRFB), is a type of rechargeable flow battery. It employs vanadium ions as charge carriers. The battery uses vanadium's ability to exist in a solution in four different oxidation states to make a battery with a single electroactive element instead of two. For several reasons, including their relative bulkiness, vanadium batteries are typically used for grid energy storage, i.e., attached to power plants/electrical grids.

A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides and in opposite direction of a membrane. Ion transfer inside the cell occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the electrodes.

Buckypaper is a thin sheet made from an aggregate of carbon nanotubes or carbon nanotube grid paper. The nanotubes are approximately 50,000 times thinner than a human hair. Originally, it was fabricated as a way to handle carbon nanotubes, but it is also being studied and developed into applications by several research groups, showing promise as vehicle armor, personal armor, and next-generation electronics and displays.
Nanodot can refer to several technologies which use nanometer-scale localized structures. Nanodots generally exploit properties of quantum dots to localize magnetic or electrical fields at very small scales. Applications for nanodots could include high-density information storage, energy storage, and light-emitting devices.
The polysulfide–bromine battery, is a type of rechargeable electric battery, which stores electric energy in liquids, such as water-based solutions of two salts: sodium bromide and sodium polysulfide. It is an example and type of redox (reduction–oxidation) flow battery.

Lithium cobalt oxide, sometimes called lithium cobaltate or lithium cobaltite, is a chemical compound with formula LiCoO
2. The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide.

A lithium-ion capacitor is a hybrid type of capacitor classified as a type of supercapacitor. It is called a hybrid because the anode is the same as those used in lithium-ion batteries and the cathode is the same as those used in supercapacitors. Activated carbon is typically used as the cathode. The anode of the LIC consists of carbon material which is often pre-doped with lithium ions. This pre-doping process lowers the potential of the anode and allows a relatively high output voltage compared to other supercapacitors.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte.
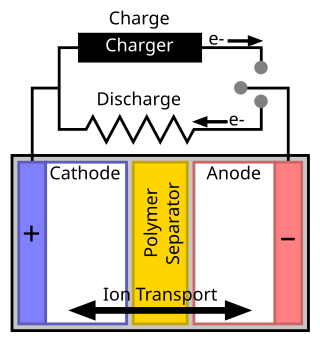
A separator is a permeable membrane placed between a battery's anode and cathode. The main function of a separator is to keep the two electrodes apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current in an electrochemical cell.

Zinc–cerium batteries are a type of redox flow battery first developed by Plurion Inc. (UK) during the 2000s. In this rechargeable battery, both negative zinc and positive cerium electrolytes are circulated though an electrochemical flow reactor during the operation and stored in two separated reservoirs. Negative and positive electrolyte compartments in the electrochemical reactor are separated by a cation-exchange membrane, usually Nafion (DuPont). The Ce(III)/Ce(IV) and Zn(II)/Zn redox reactions take place at the positive and negative electrodes, respectively. Since zinc is electroplated during charge at the negative electrode this system is classified as a hybrid flow battery. Unlike in zinc–bromine and zinc–chlorine redox flow batteries, no condensation device is needed to dissolve halogen gases. The reagents used in the zinc-cerium system are considerably less expensive than those used in the vanadium flow battery.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.
Lithium hybrid organic batteries are an energy storage device that combines lithium with an organic polymer. For example, polyaniline vanadium (V) oxide (PAni/V2O5) can be incorporated into the nitroxide-polymer lithium iron phosphate battery, PTMA/LiFePO4. Together, they improve the lithium ion intercalation capacity, cycle life, electrochemical performances, and conductivity of batteries.
Dispersed particle resistance (DPR) is a measured parameter to characterize battery active materials. It is seen as an indicator of lithium-ion battery active material rate capability. It is the slope of voltage-current linear fit for active material samples in suspensions. It can be obtained by applying different voltages on a suspension and measuring the currents, after which the data points are plotted. The slope of the plot is referred to as dispersed particle resistance. It can also be done in the opposite way where different currents are applied and voltages are measured. The key advantage of this dispersed particle resistance technique is fast and accurate comparing with the conventional characterization method for which batteries need to be fabricated and tested for a long time.

A semi-solid flow battery is a type of flow battery using solid battery active materials or involving solid species in the energy carrying fluid. A research team in MIT proposed this concept using lithium-ion battery materials. In such a system, both positive (cathode) and negative electrode (anode) consist of active material particles with carbon black suspended in liquid electrolyte. Active material suspensions are stored in two energy storage tanks. The suspensions are pumped into the electrochemical reaction cell when charging and discharging. This design takes advantage of both the designing flexibility of flow batteries and the high energy density active materials of lithium-ion batteries.

George William Crabtree was an American physicist known for his highly cited research on superconducting materials and, since 2012, for his directorship of the Joint Center for Energy Storage Research (JCESR) at Argonne National Laboratory.
Calcium (ion) batteries are energy storage and delivery technologies (i.e., electro–chemical energy storage) that employ calcium ions (cations), Ca2+, as the active charge carrier. Calcium (ion) batteries remain an active area of research, with studies and work persisting in the discovery and development of electrodes and electrolytes that enable stable, long-term battery operation.
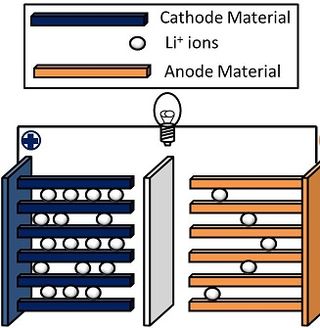
Lithium nickel manganese cobalt oxides (abbreviated NMC, Li-NMC, LNMC, or NCM) are mixed metal oxides of lithium, nickel, manganese and cobalt with the general formula LiNixMnyCo1-x-yO2. These materials are commonly used in lithium-ion batteries for mobile devices and electric vehicles, acting as the positively charged cathode.

Kenneth Ikechukwu Ozoemena is a Nigerian physical chemist, materials scientist, and academic. He is a research professor at the University of the Witwatersrand (Wits) in Johannesburg where he Heads the South African SARChI Chair in Materials Electrochemistry and Energy Technologies (MEET), supported by the Department of Science and Innovation (DSI), National Research Foundation (NRF) and Wits.











