Related Research Articles

Levonorgestrel is a hormonal medication which is used in a number of birth control methods. It is combined with an estrogen to make combination birth control pills. As an emergency birth control, sold under the brand names Plan B One-Step and Julie, among others, it is useful within 72 hours of unprotected sex. The more time that has passed since sex, the less effective the medication becomes, and it does not work after pregnancy (implantation) has occurred. Levonorgestrel works by preventing ovulation or fertilization from occurring. It decreases the chances of pregnancy by 57 to 93%. In an intrauterine device (IUD), such as Mirena among others, it is effective for the long-term prevention of pregnancy. A levonorgestrel-releasing implant is also available in some countries.
Male contraceptives, also known as male birth control, are methods of preventing pregnancy by leveraging male physiology. Globally, the most common forms of male contraceptives include condoms, vasectomy, and withdrawal. Men are largely limited to these forms of contraception, and combined, male contraceptives make up less than one-third of total contraceptive use today. The most commonly used method is the male condom.

A hormonal intrauterine device (IUD), also known as an intrauterine system (IUS) with progestogen and sold under the brand name Mirena among others, is an intrauterine device that releases a progestogenic hormonal agent such as levonorgestrel into the uterus. It is used for birth control, heavy menstrual periods, and to prevent excessive build of the lining of the uterus in those on estrogen replacement therapy. It is one of the most effective forms of birth control with a one-year failure rate around 0.2%. The device is placed in the uterus and lasts three to eight years. Fertility often returns quickly following removal.

Hormonal contraception refers to birth control methods that act on the endocrine system. Almost all methods are composed of steroid hormones, although in India one selective estrogen receptor modulator is marketed as a contraceptive. The original hormonal method—the combined oral contraceptive pill—was first marketed as a contraceptive in 1960. In the ensuing decades many other delivery methods have been developed, although the oral and injectable methods are by far the most popular. Hormonal contraception is highly effective: when taken on the prescribed schedule, users of steroid hormone methods experience pregnancy rates of less than 1% per year. Perfect-use pregnancy rates for most hormonal contraceptives are usually around the 0.3% rate or less. Currently available methods can only be used by women; the development of a male hormonal contraceptive is an active research area.

The Concept Foundation is a non-profit foundation which was established by the UNDP/UNFPA/WHO/WB Special program in Reproductive Health (WHO/HRP), PATH, the World Bank in 1989 in Bangkok, Thailand, "as a mechanism through which WHO’s rights associated with an injectable contraceptive, Cyclofem, could be licensed to potential producers in developing countries". Estradiol cypionate/medroxyprogesterone acetate, is a once-a-month combined injectable contraceptive which contains 25 mg of medroxyprogesterone acetate—the same ingredient in Depo Provera—and 5 mg of estradiol cypionate.
Combined injectable contraceptives (CICs) are a form of hormonal birth control for women. They consist of monthly injections of combined formulations containing an estrogen and a progestin to prevent pregnancy.

Algestone acetophenide, also known more commonly as dihydroxyprogesterone acetophenide (DHPA) and sold under the brand names Perlutal and Topasel among others, is a progestin medication which is used in combination with an estrogen as a form of long-lasting injectable birth control. It has also been used alone, but is no longer available as a standalone medication. DHPA is not active by mouth and is given once a month by injection into muscle.

Norethisterone enanthate (NETE), also known as norethindrone enanthate, is a form of hormonal birth control which is used to prevent pregnancy in women. It is used both as a form of progestogen-only injectable birth control and in combined injectable birth control formulations. It may be used following childbirth, miscarriage, or abortion. The failure rate per year in preventing pregnancy for the progestogen-only formulation is 2 per 100 women. Each dose of this form lasts two months with only up to two doses typically recommended.
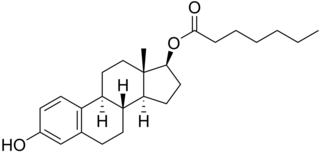
Estradiol enantate, also spelled estradiol enanthate and sold under the brand names Perlutal and Topasel among others, is an estrogen medication which is used in hormonal birth control for women. It is formulated in combination with dihydroxyprogesterone acetophenide, a progestin, and is used specifically as a combined injectable contraceptive. Estradiol enantate is not available for medical use alone. The medication, in combination with DHPA, is given by injection into muscle once a month.
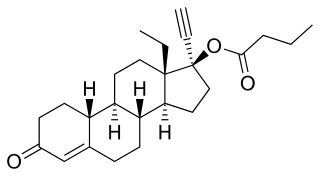
Levonorgestrel butanoate (LNG-B), or levonorgestrel 17β-butanoate, is a steroidal progestin of the 19-nortestosterone group which was developed by the World Health Organization (WHO) in collaboration with the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development as a long-acting injectable contraceptive. It is the C17β butanoate ester of levonorgestrel, and acts as a prodrug of levonorgestrel in the body. The drug is at or beyond the phase III stage of clinical development, but has not been marketed at this time. It was first described in the literature, by the WHO, in 1983, and has been under investigation for potential clinical use since then.

Estradiol cypionate/medroxyprogesterone acetate (EC/MPA), sold under the brand name Cyclofem among others, is a form of combined injectable birth control. It contains estradiol cypionate (EC), an estrogen, and medroxyprogesterone acetate (MPA), a progestin. It is recommended for short-term use and is given once a month by injection into a muscle.

Estradiol valerate/norethisterone enantate (EV/NETE), sold under the brand name Mesigyna among others, is a form of combined injectable birth control which is used to prevent pregnancy in women. It contains estradiol valerate (EV), an estrogen, and norethisterone enantate (NETE), a progestin. The medication is given once a month by injection into muscle.

Estradiol enantate/algestone acetophenide, also known as estradiol enantate/dihydroxyprogesterone acetophenide (E2-EN/DHPA) and sold under the brand names Perlutal and Topasel among others, is a form of combined injectable birth control which is used to prevent pregnancy. It contains estradiol enantate (E2-EN), an estrogen, and algestone acetophenide, a progestin. The medication is given once a month by injection into muscle.

Estradiol benzoate butyrate/algestone acetophenide, also known as estradiol benzoate butyrate/dihydroxyprogesterone acetophenide (EBB/DHPA) and sold under the brand names Neolutin N, Redimen, Soluna, and Unijab, is a form of combined injectable birth control which is used in Peru and Singapore. It contains estradiol benzoate butyrate (EBB), an estrogen, and algestone acetophenide, a progestin. The medication is given once per month by injection into muscle.

Chlormadinone caproate (CMC) is a progestin and a progestogen ester which was studied for potential use in combined injectable contraceptives but was never marketed. It was assessed in combination with estradiol valerate at doses of 80 mg and 3 mg, respectively. In addition to chlormadinone acetate (CMA), analogues of CMC include gestonorone caproate, hydroxyprogesterone caproate, medroxyprogesterone caproate, megestrol caproate, and methenmadinone caproate.

Levonorgestrel cyclobutylcarboxylate is a progestin and a progestogen ester which was studied for potential use as an injectable hormonal contraceptive but was never marketed. It was developed by the World Health Organization's Special Programme on Human Reproduction in the 1980s. Analogues of levonorgestrel cyclobutylcarboxylate include levonorgestrel butanoate (HRP-002) and levonorgestrel cyclopropylcarboxylate (HRP-003).

Levonorgestrel cyclopropylcarboxylate, or levonorgestrel 17β-cyclopropylcarboxylate, is a progestin and a progestogen ester which was studied for potential use as an injectable hormonal contraceptive but was never marketed. It was developed by the World Health Organization's Special Programme on Human Reproduction in the 1980s. Analogues of levonorgestrel cyclopropylcarboxylate include levonorgestrel cyclobutylcarboxylate (HRP-001) and levonorgestrel butanoate (HRP-002).
The Human Reproduction Program -UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) is a WHO co-sponsored research programme on human reproduction. HRP is based at the WHO headquarters in Geneva, Switzerland. Its goal is to support and coordinate research on a global scale. It is part of the Sexual and Reproductive Health and Research (SRH) department of the WHO.

Katayun Dattatraya Virkar was an Indian physician and medical researcher, head of the contraception division at India's National Institute of Research on Reproductive Health (NIRRH).
References
- ↑ "sexual and reproductive health and research-SHR" . Retrieved 9 March 2023.
- 1 2 Benagiano, G., & Merialdi, M. (2011). Carl Djerassi and the World Health Organisation special programme of research in human reproduction. Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology, 8(1), 10-13. http://www.kup.at/kup/pdf/10163.pdf
- 1 2 Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- 1 2 Koetsawang S (1991). "The injectable contraceptive: present and future trends". Ann. N. Y. Acad. Sci. 626 (1): 30–42. Bibcode:1991NYASA.626...30K. doi:10.1111/j.1749-6632.1991.tb37897.x. PMID 1829341. S2CID 27008012.
- 1 2 Garza-Flores J, Hall PE, Perez-Palacios G (1991). "Long-acting hormonal contraceptives for women". J. Steroid Biochem. Mol. Biol. 40 (4–6): 697–704. doi:10.1016/0960-0760(91)90293-E. PMID 1958567. S2CID 26021562.
- ↑ Koetsawang S (April 1994). "Once-a-month injectable contraceptives: efficacy and reasons for discontinuation". Contraception. 49 (4): 387–98. doi:10.1016/0010-7824(94)90034-5. PMID 8013221.
- ↑ Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- ↑ Fraser IS (April 1994). "Vaginal bleeding patterns in women using once-a-month injectable contraceptives". Contraception. 49 (4): 399–420. doi:10.1016/0010-7824(94)90035-3. PMID 8013222.
- ↑ Hassan EO, el-Nahal N, el-Hussinie M (August 1999). "Once-a-month injectable contraceptives, Cyclofem and Mesigyna, in Egypt. Efficacy, causes of discontinuation, and side effects". Contraception. 60 (2): 87–92. doi:10.1016/S0010-7824(99)00064-5. PMID 10592855.
- ↑ Giwa-Osagie OF (May 1994). "Metabolic effects of once-a-month combined injectable contraceptives. The World Health Organization Task Force on Long-Acting Systemic Agents for Fertility Regulation, Geneva, Switzerland". Contraception. 49 (5): 421–33. doi:10.1016/0010-7824(94)90001-9. PMID 8045129.
- ↑ "Population growth and economic development". United Nations. March 9, 1963.
- 1 2 "Decisions of the United Nations, specialized agencies and the International Atomic Energy Agency affecting WHO's activities (programme matters): programme activities in the health aspects of world population which might be developed by WHO: report by the Director-General". March 9, 1965. hdl:10665/136768.
- ↑ "Decisions of the United Nations affecting WHO's activities: resolution 1048 (XXXVII) of the Economic and Social Council". March 9, 1965. hdl:10665/88958.
- ↑ "Programme activities in the health aspects of world population which might be developed by WHO". March 9, 1965. hdl:10665/89329.
- ↑ Candau, Marcolino Gomes (March 9, 1971). The work of WHO, 1970: annual report of the Director-General to the World Health Assembly and to the United Nations. World Health Organization. hdl: 10665/85828 . ISBN 9241601884.
- ↑ "Health aspects of population dynamics". March 9, 1969. hdl:10665/91257.
- ↑ "World Health Organization: Expanded Programme of Research, Development and Research Training in Human Reproduction: Report of a Feasibility Project, May 1971; HR/71.4 (1971) part 1" (PDF). hdl:10665/361192.
- ↑ "World Health Organization: Resolution WHA25.60 on WHO's role in the development and coordination of biomedical research, Twenty-fifth World Health Assembly, Geneva, 9-26 May 1972 - Part I: resolutions and decisions pp.32-33". 26 May 1972. Retrieved 13 March 2023.
- ↑ "World Health Organization: EB51/6 - WHO's role in the development and coordination of biomedical research: interim report by the Director-General, December 1972" (PDF). 20 December 1972. Retrieved 13 March 2023.
- 1 2 Mbizvo, Michael T.; d'Arcangues, Catherine; Van Look, Paul FA; Benagiano, Giuseppe (2012). "40 years of innovation in sexual and reproductive health". The Lancet. 380 (9843): 705–706. doi:10.1016/S0140-6736(12)61025-3. PMID 22920740. S2CID 205966657.
- ↑ "Expanded Programme of Research, Development and Research Training in Human Reproduction: report of a feasibility project: supplementary information". March 9, 1971. hdl:10665/361193.
- ↑ "World Health Organization: EM/RC33/13 - Nomination of a member state to the Policy and Coordination advisory committee (PCAC) of the Special programme of research, development, and research training in human reproduction (HRP), July 1986" (PDF). hdl:10665/120636.
- 1 2 "RECENT DEVELOPMENTS IN HUMAN REPRODUCTION RESEARCH - Progress report by the Director-General" (PDF). hdl:10665/162389.
- ↑ Khosla, Rajat; Amin, Avni; Allotey, Pascale; Barroso, Carmen; George, Asha; Hardon, Anita; Askew, Ian (January 1, 2019). ""Righting the wrongs": addressing human rights and gender equality through research since Cairo". Sexual and Reproductive Health Matters. 27 (1): 329–332. doi:10.1080/26410397.2019.1676529. PMC 7887975 . PMID 31746277.
- ↑ "Special Programmes: UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP): Policy and Coordination Committee (PCC) – Report on attendance at PCC in 2020 and nomination of a Member in place of Bhutan whose term expires on 31 December 2020". March 9, 2020. hdl:10665/333620.