
A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.

Ketone bodies are water-soluble molecules that contain the ketone groups produced from fatty acids by the liver (ketogenesis). They are readily transported into tissues outside the liver, where they are converted into acetyl-CoA —which then enters the citric acid cycle and is oxidized for energy. Ketone bodies in the brain are used to convert acetyl-CoA into long-chain fatty acids. These liver-derived ketone groups include acetoacetic acid (acetoacetate), beta-hydroxybutyrate, and acetone, a spontaneous breakdown product of acetoacetate
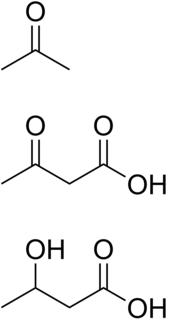
Ketosis is a metabolic state characterized by elevated levels of ketone bodies in the blood or urine. Physiologic ketosis is a normal response to low glucose availability, such as low-carbohydrate diets or fasting, that provides an additional energy source for the brain in the form of ketones. In physiologic ketosis, ketones in the blood are elevated above baseline levels, but the body's acid–base homeostasis is maintained. This contrasts with ketoacidosis, an uncontrolled production of ketones that occurs in pathologic states and causes a metabolic acidosis, which is a medical emergency. Ketoacidosis is most commonly the result of complete insulin deficiency in type 1 diabetes or late-stage type 2 diabetes. Ketone levels can be measured in blood, urine or breath and are generally between 0.5 and 3.0 millimolar (mM) in physiologic ketosis, while ketoacidosis may cause blood concentrations greater than 10 mM.

Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.

Ketogenesis is the biochemical process through which organisms produce ketone bodies by breaking down fatty acids and ketogenic amino acids. The process supplies energy to certain organs, particularly the brain, heart and skeletal muscle, under specific scenarios including fasting, caloric restriction, sleep, or others.

An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone.

Ketoacidosis is a metabolic state caused by uncontrolled production of ketone bodies that cause a metabolic acidosis. While ketosis refers to any elevation of blood ketones, ketoacidosis is a specific pathologic condition that results in changes in blood pH and requires medical attention. The most common cause of ketoacidosis is diabetic ketoacidosis but can also be caused by alcohol, medications, toxins, and rarely starvation.
Enols, or more formally, alkenols, are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.
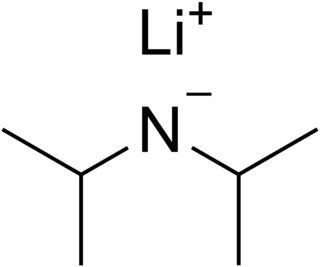
Lithium diisopropylamide is a chemical compound with the molecular formula [(CH3)2CH]2NLi. It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group.

3-Hydroxy-3-methylglutaryl-CoA lyase deficiency is an uncommon inherited disorder in which the body cannot properly process the amino acid leucine. Additionally, the disorder prevents the body from making ketones, which are used for energy during fasting.

Ketonuria is a medical condition in which ketone bodies are present in the urine.

Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid with a pungent odor. It is soluble in water and polar organic solvents. It is a useful intermediate in the synthesis of other compounds.

Raspberry ketone is a natural phenolic compound that is the primary aroma compound of red raspberries.

Buphedrone, also known as α-methylamino-butyrophenone (MABP), is a stimulant of the phenethylamine and cathinone chemical classes that was first synthesized in 1928. It is legal in most countries as a research chemical, as long as it is not intended for human consumption.
Medroxalol is a vasodilator beta blocker also classified as a mixed receptor blocker as it blocks both alpha and beta receptors.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.
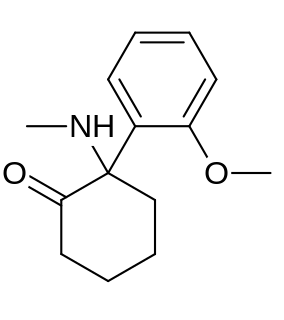
Methoxyketamine or 2-MeO-2-deschloroketamine is a designer drug of the arylcyclohexylamine class first reported in 1963. It is an analog of ketamine in which the chlorine atom has been replaced with a methoxy group. Its synthesis by rearrangement of an amino ketone has been reported. As an arylcyclohexylamine, methoxyketamine most likely functions as an NMDA receptor antagonist. It produces sedative, hallucinogenic, and anesthetic effects, but with a lower potency than ketamine itself.

A telluroketone is an analog of a ketone in which the oxygen atom has been replaced by a tellurium atom. This change makes the functional group less stable, requiring greater steric and electronic stabilization.

Bromomethyl ethyl ketone is a brominated ketone with lachrymatory effects. It was used as a chemical warfare agent in World War I. Bromomethyl ethyl ketone was developed as an alternative to bromoacetone, because acetone, the precursor to bromoacetone, was required for explosives production.

















