
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.

In thermodynamics, the enthalpy of vaporization, also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. The enthalpy of vaporization is a function of the pressure and temperature at which the transformation takes place.

The melting point of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.

Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.

Convection is single or multiphase fluid flow that occurs spontaneously through the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity. When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow.

Josef Stefan was a Carinthian Slovene physicist, mathematician, and poet of the Austrian Empire.
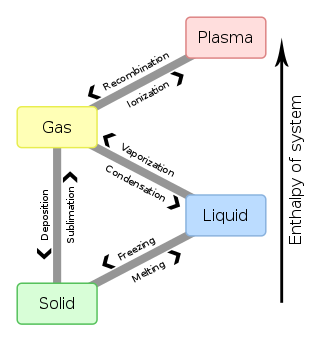
In physics, chemistry, and other related fields like biology, a phase transition is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point.
Sensible heat is heat exchanged by a body or thermodynamic system in which the exchange of heat changes the temperature of the body or system, and some macroscopic variables of the body or system, but leaves unchanged certain other macroscopic variables of the body or system, such as volume or pressure.

Latent heat is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation.

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.

Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point.

Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. As per the established international definition, supercooling means ‘cooling a substance below the normal freezing point without solidification’ While it can be achieved by different physical means, the postponed solidification is most often due to the absence of seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−54.9 °F). Supercooled water can occur naturally, for example in the atmosphere, animals or plants.
In mathematics and its applications, particularly to phase transitions in matter, a Stefan problem is a particular kind of boundary value problem for a system of partial differential equations (PDE), in which the boundary between the phases can move with time. The classical Stefan problem aims to describe the evolution of the boundary between two phases of a material undergoing a phase change, for example the melting of a solid, such as ice to water. This is accomplished by solving heat equations in both regions, subject to given boundary and initial conditions. At the interface between the phases the temperature is set to the phase change temperature. To close the mathematical system a further equation, the Stefan condition, is required. This is an energy balance which defines the position of the moving interface. Note that this evolving boundary is an unknown (hyper-)surface; hence, Stefan problems are examples of free boundary problems.
The Clausius–Clapeyron relation, in chemical thermodynamics, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. It is named after Rudolf Clausius and Benoît Paul Émile Clapeyron. However, this relation was in fact originally derived by Sadi Carnot in his Reflections on the Motive Power of Fire, which was published in 1824 but largely ignored until it was rediscovered by Clausius, Clapeyron, and Lord Kelvin decades later. Kelvin said of Carnot's argument that "nothing in the whole range of Natural Philosophy is more remarkable than the establishment of general laws by such a process of reasoning."

A phase-change material (PCM) is a substance which releases/absorbs sufficient energy at phase transition to provide useful heat or cooling. Generally the transition will be from one of the first two fundamental states of matter - solid and liquid - to the other. The phase transition may also be between non-classical states of matter, such as the conformity of crystals, where the material goes from conforming to one crystalline structure to conforming to another, which may be a higher or lower energy state.

Thermal energy storage (TES) is the storage of thermal energy for later reuse. Employing widely different technologies, it allows surplus thermal energy to be stored for hours, days, or months. Scale both of storage and use vary from small to large – from individual processes to district, town, or region. Usage examples are the balancing of energy demand between daytime and nighttime, storing summer heat for winter heating, or winter cold for summer cooling. Storage media include water or ice-slush tanks, masses of native earth or bedrock accessed with heat exchangers by means of boreholes, deep aquifers contained between impermeable strata; shallow, lined pits filled with gravel and water and insulated at the top, as well as eutectic solutions and phase-change materials.

Regelation is the phenomenon of ice melting under pressure and refreezing when the pressure is reduced. This can be demonstrated by looping a fine wire around a block of ice, with a heavy weight attached to it. The pressure exerted on the ice slowly melts it locally, permitting the wire to pass through the entire block. The wire's track will refill as soon as pressure is relieved, so the ice block will remain intact even after wire passes completely through. This experiment is possible for ice at −10 °C or cooler, and while essentially valid, the details of the process by which the wire passes through the ice are complex. The phenomenon works best with high thermal conductivity materials such as copper, since latent heat of fusion from the top side needs to be transferred to the lower side to supply latent heat of melting. In short, the phenomenon in which ice converts to liquid due to applied pressure and then re-converts to ice once the pressure is removed is called regelation.

Thermodynamic databases contain information about thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free energy. Numerical values of these thermodynamic properties are collected as tables or are calculated from thermodynamic datafiles. Data is expressed as temperature-dependent values for one mole of substance at the standard pressure of 101.325 kPa, or 100 kPa. Both of these definitions for the standard condition for pressure are in use.

In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a solid to a liquid, at constant pressure.
In mathematics, a free boundary problem is a partial differential equation to be solved for both an unknown function and an unknown domain . The segment of the boundary of which is not known at the outset of the problem is the free boundary.














