Ionization or ionisation, is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.

The Geiger–Müller tube or G–M tube is the sensing element of the Geiger counter instrument used for the detection of ionizing radiation. It was named after Hans Geiger, who invented the principle in 1908, and Walther Müller, who collaborated with Geiger in developing the technique further in 1928 to produce a practical tube that could detect a number of different radiation types.

A corona discharge is an electrical discharge brought on by the ionization of a fluid such as air surrounding a conductor that is electrically charged. Spontaneous corona discharges occur naturally in high-voltage systems unless care is taken to limit the electric field strength. A corona will occur when the strength of the electric field around a conductor is high enough to form a conductive region, but not high enough to cause electrical breakdown or arcing to nearby objects. It is often seen as a bluish glow in the air adjacent to pointed metal conductors carrying high voltages, and emits light by the same property as a gas discharge lamp.
A helium–neon laser or HeNe laser, is a type of gas laser whose gain medium consists of a mixture of 75% helium and 25% neon at a total pressure of about 1 mm of Hg inside of a small electrical discharge. The best-known and most widely used HeNe laser operates at a wavelength of 632.8 nm, in the red part of the visible spectrum.

The Franck–Hertz experiment was the first electrical measurement to clearly show the quantum nature of atoms, and thus "transformed our understanding of the world". It was presented on April 24, 1914, to the German Physical Society in a paper by James Franck and Gustav Hertz. Franck and Hertz had designed a vacuum tube for studying energetic electrons that flew through a thin vapor of mercury atoms. They discovered that, when an electron collided with a mercury atom, it could lose only a specific quantity of its kinetic energy before flying away. This energy loss corresponds to decelerating the electron from a speed of about 1.3 million meters per second to zero. A faster electron does not decelerate completely after a collision, but loses precisely the same amount of its kinetic energy. Slower electrons merely bounce off mercury atoms without losing any significant speed or kinetic energy.

A flashtube, also called a flashlamp, is an electric arc lamp designed to produce extremely intense, incoherent, full-spectrum white light for very short durations. Flashtubes are made of a length of glass tubing with electrodes at either end and are filled with a gas that, when triggered, ionizes and conducts a high voltage pulse to produce the light. Flashtubes are used mostly for photographic purposes but are also employed in scientific, medical, industrial, and entertainment applications.

A gas-filled tube, also known as a discharge tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric discharge in gases, and operate by ionizing the gas with an applied voltage sufficient to cause electrical conduction by the underlying phenomena of the Townsend discharge. A gas-discharge lamp is an electric light using a gas-filled tube; these include fluorescent lamps, metal-halide lamps, sodium-vapor lamps, and neon lights. Specialized gas-filled tubes such as krytrons, thyratrons, and ignitrons are used as switching devices in electric devices.

Pulsed laser deposition (PLD) is a physical vapor deposition (PVD) technique where a high-power pulsed laser beam is focused inside a vacuum chamber to strike a target of the material that is to be deposited. This material is vaporized from the target which deposits it as a thin film on a substrate. This process can occur in ultra high vacuum or in the presence of a background gas, such as oxygen which is commonly used when depositing oxides to fully oxygenate the deposited films.

A glow discharge is a plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a value called the striking voltage, the gas ionization becomes self-sustaining, and the tube glows with a colored light. The color depends on the gas used.

Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules are ionized by electron ionization, which subsequently react with analyte molecules in the gas phase in order to achieve ionization. Negative chemical ionization (NCI), charge-exchange chemical ionization and atmospheric-pressure chemical ionization (APCI) are some of the common variations of this technique. CI has several important applications in identification, structure elucidation and quantitation of organic compounds. Beside the applications in analytical chemistry, the usefulness in chemical ionization extends toward biochemical, biological and medicinal fields as well.

An ion laser is a gas laser that uses an ionized gas as its lasing medium. Like other gas lasers, ion lasers feature a sealed cavity containing the laser medium and mirrors forming a Fabry–Pérot resonator. Unlike helium–neon lasers, the energy level transitions that contribute to laser action come from ions. Because of the large amount of energy required to excite the ionic transitions used in ion lasers, the required current is much greater, and as a result all but the smallest ion lasers are water-cooled. A small air-cooled ion laser might produce, for example, 130 milliwatts of output light with a tube current of about 10 amperes and a voltage of 105 volts. Since one ampere times one volt is one watt, this is an electrical power input of about one kilowatt. Subtracting the (desirable) light output of 130 mW from power input, this leaves the large amount of waste heat of nearly one kW. This has to be dissipated by the cooling system. In other words, the power efficiency is very low.

A nitrogen laser is a gas laser operating in the ultraviolet range using molecular nitrogen as its gain medium, pumped by an electrical discharge.

A residual gas analyzer (RGA) is a small and usually rugged mass spectrometer, typically designed for process control and contamination monitoring in vacuum systems. Utilizing quadrupole technology, there exists two implementations, utilizing either an open ion source (OIS) or a closed ion source (CIS). RGAs may be found in high vacuum applications such as research chambers, surface science setups, accelerators, scanning microscopes, etc. RGAs are used in most cases to monitor the quality of the vacuum and easily detect minute traces of impurities in the low-pressure gas environment. These impurities can be measured down to Torr levels, possessing sub-ppm detectability in the absence of background interferences.

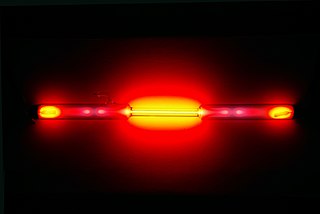
Gas-discharge lamps are a family of artificial light sources that generate light by sending an electric discharge through an ionized gas, a plasma. Typically, such lamps use a noble gas or a mixture of these gases. Some include additional substances, like mercury, sodium, and metal halides, which are vaporized during startup to become part of the gas mixture. In operation, some of the electrons are forced to leave the atoms of the gas near the anode by the electric field applied between the two electrodes, leaving these atoms positively ionized. The free electrons thus released flowing onto the anode, while the cations thus formed are accelerated by the electric field and flow towards the cathode. Typically, after traveling a very short distance, the ions collide with neutral gas atoms, which transfer their electrons to the ions. The atoms, having lost an electron during the collisions, ionize and speed toward the cathode while the ions, having gained an electron during the collisions, return to a lower energy state while releasing energy in the form of photons. Light of a characteristic frequency is thus emitted. In this way, electrons are relayed through the gas from the cathode to the anode. The color of the light produced depends on the emission spectra of the atoms making up the gas, as well as the pressure of the gas, current density, and other variables. Gas discharge lamps can produce a wide range of colors. Some lamps produce ultraviolet radiation which is converted to visible light by a fluorescent coating on the inside of the lamp's glass surface. The fluorescent lamp is perhaps the best known gas-discharge lamp.
A TEA laser is a gas laser energized by a high voltage electrical discharge in a gas mixture generally at or above atmospheric pressure. The most common types are carbon dioxide lasers and excimer lasers, both used extensively in industry and research, less common are nitrogen lasers. The acronym "TEA" stands for Transversely Excited Atmospheric.
A Penning mixture, named after Frans Michel Penning, is a mixture of gases used in electric lighting or displaying fixtures. Although the popular phrase for the most common of these is a neon lamp, it is more efficient to have the glass tube filled not with pure neon, but with a Penning mixture, which is defined as a mixture of one inert gas with a minute amount of another gas, one that has lower ionization voltage than the main constituent.
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is put to practical use in applications such as gas-discharge neon lamps and fluorescent lamps, where the lamp is filled with a Penning mixture to improve the electrical characteristics of the lamps.

Plasma-enhanced chemical vapor deposition (PECVD) is a chemical vapor deposition process used to deposit thin films from a gas state (vapor) to a solid state on a substrate. Chemical reactions are involved in the process, which occur after creation of a plasma of the reacting gases. The plasma is generally created by radio frequency (RF) frequency or direct current (DC) discharge between two electrodes, the space between which is filled with the reacting gases.
Electric discharge in gases occurs when electric current flows through a gaseous medium due to ionization of the gas. Depending on several factors, the discharge may radiate visible light. The properties of electric discharges in gases are studied in connection with design of lighting sources and in the design of high voltage electrical equipment.
Xenon monochloride (XeCl) is an excimer which is used in excimer lasers emitting near ultraviolet light at 308 nm. It is most commonly used in medicine. It was used to produce a body image from the Shroud of Turin. The results suggest that a directional burst of ultraviolet radiation may have played a role in the formation of the image on the Shroud.