Luteinizing hormone is a hormone produced by gonadotropic cells in the anterior pituitary gland. The production of LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus. In females, an acute rise of LH known as an LH surge, triggers ovulation and development of the corpus luteum. In males, where LH had also been called interstitial cell–stimulating hormone (ICSH), it stimulates Leydig cell production of testosterone. It acts synergistically with follicle-stimulating hormone (FSH).
A hormone receptor is a receptor molecule that binds to a specific hormone. Hormone receptors are a wide family of proteins made up of receptors for thyroid and steroid hormones, retinoids and Vitamin D, and a variety of other receptors for various ligands, such as fatty acids and prostaglandins. Hormone receptors are of mainly two classes. Receptors for peptide hormones tend to be cell surface receptors built into the plasma membrane of cells and are thus referred to as trans membrane receptors. An example of this is Actrapid. Receptors for steroid hormones are usually found within the protoplasm and are referred to as intracellular or nuclear receptors, such as testosterone. Upon hormone binding, the receptor can initiate multiple signaling pathways, which ultimately leads to changes in the behavior of the target cells.
Gonadotropin-releasing hormone (GnRH) is a releasing hormone responsible for the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. GnRH is a tropic peptide hormone synthesized and released from GnRH neurons within the hypothalamus. GnRH is inhibited by testosterone. The peptide belongs to gonadotropin-releasing hormone family. It constitutes the initial step in the hypothalamic–pituitary–gonadal axis.

Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effects are mediated by slow genomic mechanisms through nuclear receptors as well as by fast nongenomic mechanisms through membrane-associated receptors and signaling cascades. The polypeptide hormones luteinizing hormone, follicle-stimulating hormone and gonadotropin-releasing hormone – each associated with the gonadotropin axis – are usually not regarded as sex hormones, although they play major sex-related roles.
Gonadotropins are glycoprotein hormones secreted by gonadotropic cells of the anterior pituitary of vertebrates. This family includes the mammalian hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH), the placental/chorionic gonadotropins, human chorionic gonadotropin (hCG) and equine chorionic gonadotropin (eCG), as well as at least two forms of fish gonadotropins. These hormones are central to the complex endocrine system that regulates normal growth, sexual development, and reproductive function. LH and FSH are secreted by the anterior pituitary gland, while hCG and eCG are secreted by the placenta in pregnant women and mares, respectively. The gonadotropins act on the gonads, controlling gamete and sex hormone production.
The gonadotropin-releasing hormone receptor (GnRHR), also known as the luteinizing hormone releasing hormone receptor (LHRHR), is a member of the seven-transmembrane, G-protein coupled receptor (GPCR) family. It is the receptor of gonadotropin-releasing hormone (GnRH). Agonist binding to the GnRH receptor activates the Gq/11 family of heterotrimeric G proteins. The GnRHR is expressed on the surface of pituitary gonadotrope cells as well as lymphocytes, breast, ovary, and prostate.
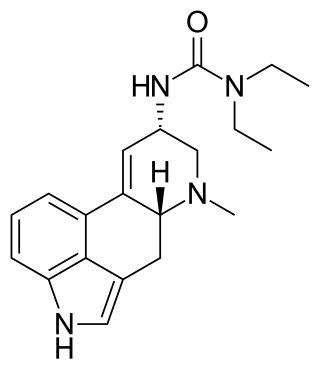
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline class which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels. It is taken by mouth.

Neurotensin is a 13 amino acid neuropeptide that is implicated in the regulation of luteinizing hormone and prolactin release and has significant interaction with the dopaminergic system. Neurotensin was first isolated from extracts of bovine hypothalamus based on its ability to cause a visible vasodilation in the exposed cutaneous regions of anesthetized rats.
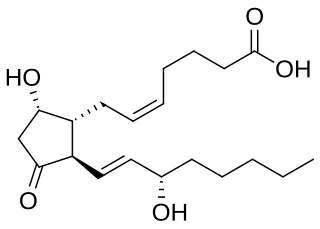
Prostaglandin D2 (or PGD2) is a prostaglandin that binds to the receptor PTGDR (DP1), as well as CRTH2 (DP2). It is a major prostaglandin produced by mast cells – recruits Th2 cells, eosinophils, and basophils. In mammalian organs, large amounts of PGD2 are found only in the brain and in mast cells. It is critical to development of allergic diseases such as asthma. Research carried out in 1989 found PGD2 is the primary mediator of vasodilation (the "niacin flush") after ingestion of niacin (nicotinic acid).

Gonadotropin-releasing hormone receptor is a protein that in humans is encoded by the GNRHR gene.

G-protein coupled receptor 146 is a protein that in humans is encoded by the GPR146 gene. The receptor has been shown to bind cholesin/C17orf50, a gut-derived hormone that is secreted from the intestine in response to dietary cholesterol absorption. In response to cholesin binding, GPR146 signaling inhibit cholesterol synthesis. Consistent with this interaction, murine genetic disruption of GPR146 lowers serum cholesterol and reduces atherosclerotic aortic lesions. GPR146 has also been identified as a possible receptor for C-peptide.
Epileptogenesis is the gradual process by which a typical brain develops epilepsy. Epilepsy is a chronic condition in which seizures occur. These changes to the brain occasionally cause neurons to fire in an abnormal, hypersynchronous manner, known as a seizure.

A HEAT repeat is a protein tandem repeat structural motif composed of two alpha helices linked by a short loop. HEAT repeats can form alpha solenoids, a type of solenoid protein domain found in a number of cytoplasmic proteins. The name "HEAT" is an acronym for four proteins in which this repeat structure is found: Huntingtin, elongation factor 3 (EF3), protein phosphatase 2A (PP2A), and the yeast kinase TOR1. HEAT repeats form extended superhelical structures which are often involved in intracellular transport; they are structurally related to armadillo repeats. The nuclear transport protein importin beta contains 19 HEAT repeats.
Infertility in polycystic ovary disease (PCOS) is a hormonal imbalance in women that is thought to be one of the leading causes of female infertility. Polycystic ovary syndrome causes more than 75% of cases of anovulatory infertility.
Sérgio A. Lira, is a Brazilian-born American immunologist who pioneered the use of genetic approaches to study the function of chemokines. His early studies were the first to show that chemokines played a major role on leukocyte trafficking to the brain, the lung and the thymus.
Mladen Vranic, MD, DSc, O.C., O.Ont, FRSC, FRCP(C), FCAHS, Canadian Medical Hall of Fame[CMHF] April 3, 1930 – June 18, 2019, was a Croatian-born diabetes researcher, best known for his work in tracer methodology, exercise and stress in diabetes, the metabolic effects of hormonal interactions, glucagon physiology, extrapancreatic glucagon, the role of the direct and indirect metabolic effects of insulin and the prevention of hypoglycemia. Vranic was recognized by a number of national and international awards for his research contributions, mentoring and administration including the Orders of Canada (Officer) and Ontario.
Chloride channel openers refer to a specific category of drugs designed to modulate chloride channels in the human body. Chloride channels are anion-selective channels which are involved in a wide variety of physiological functions and processes such as the regulation of neuroexcitation, transepithelial salt transport, and smooth muscle contraction. Due to their distribution throughout the body, diversity, functionality, and associated pathology, chloride channels represent an ideal target for the development of channel modulating drugs such as chloride channel openers.

Marta Filizola is a computational biophysicist who studies membrane proteins. Filizola's research concerns drug discovery the application of methods of computational chemistry and theoretical chemistry to biochemical and biomedical problems.
Glutamate-rich protein 4 is encoded by the gene ERICH4 and can be otherwise known as chromosome 19 open reading frame 69 (C19orf69). ERICH4 is highly conserved in mammals and exhibits overexpression in tissues of the kidneys, terminal ileum, and duodenum. The function of ERICH4 has yet to be well understood by the scientific community but is suggested to contribute to immune inflammatory responses.









