Related Research Articles

Radiocarbon dating is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon.
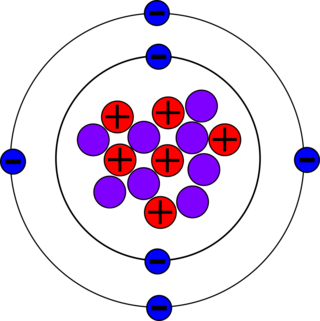
Carbon-14, C-14, 14
C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934.

Isotope analysis is the identification of isotopic signature, abundance of certain stable isotopes of chemical elements within organic and inorganic compounds. Isotopic analysis can be used to understand the flow of energy through a food web, to reconstruct past environmental and climatic conditions, to investigate human and animal diets, for food authentification, and a variety of other physical, geological, palaeontological and chemical processes. Stable isotope ratios are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio.
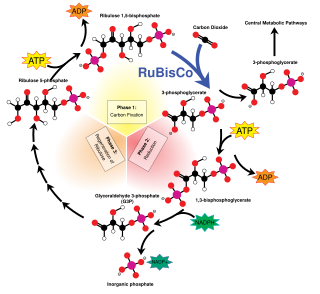
C3 carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, the other two being C4 and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phosphoglycerate through the following reaction:
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth.
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope-ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them.
Isotopomers or isotopic isomers are isomers which differ by isotopic substitution, and which have the same number of atoms of each isotope but in a different arrangement. For example, CH3OD and CH2DOH are two isotopomers of monodeuterated methanol.
Kinetic fractionation is an isotopic fractionation process that separates stable isotopes from each other by their mass during unidirectional processes. Biological processes are generally unidirectional and are very good examples of "kinetic" isotope reactions. All organisms preferentially use lighter isotopic species, because "energy costs" are lower, resulting in a significant fractionation between the substrate (heavier) and the biologically mediated product (lighter). As an example, photosynthesis preferentially takes up the light isotope of carbon 12C during assimilation of an atmospheric CO2 molecule. This kinetic isotope fractionation explains why plant material (and thus fossil fuels, which are derived from plants) is typically depleted in 13C by 25 per mil (2.5 per cent) relative to most inorganic carbon on Earth.
Carbon (6C) has 14 known isotopes, from 8
C
to 20
C
as well as 22
C
, of which 12
C
and 13
C
are stable. The longest-lived radioisotope is 14
C
, with a half-life of 5.70(3)×103 years. This is also the only carbon radioisotope found in nature, as trace quantities are formed cosmogenically by the reaction 14
N
+
n
→ 14
C
+ 1
H
. The most stable artificial radioisotope is 11
C
, which has a half-life of 20.3402(53) min. All other radioisotopes have half-lives under 20 seconds, most less than 200 milliseconds. The least stable isotope is 8
C
, with a half-life of 3.5(1.4)×10−21 s. Light isotopes tend to decay into isotopes of boron and heavy ones tend to decay into isotopes of nitrogen.
An isotopic signature is a ratio of non-radiogenic 'stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample material are measured by isotope-ratio mass spectrometry against an isotopic reference material. This process is called isotope analysis.

Soil respiration refers to the production of carbon dioxide when soil organisms respire. This includes respiration of plant roots, the rhizosphere, microbes and fauna.
In geochemistry, paleoclimatology, and paleoceanography δ13C is an isotopic signature, a measure of the ratio of the two stable isotopes of carbon—13C and 12C—reported in parts per thousand. The measure is also widely used in archaeology for the reconstruction of past diets, particularly to see if marine foods or certain types of plants were consumed.

The atmospheric carbon cycle accounts for the exchange of gaseous carbon compounds, primarily carbon dioxide, between Earth's atmosphere, the oceans, and the terrestrial biosphere. It is one of the faster components of the planet's overall carbon cycle, supporting the exchange of more than 200 billion tons of carbon in and out of the atmosphere throughout the course of each year. Atmospheric concentrations of CO2 remain stable over longer timescales only when there exists a balance between these two flows. Methane, Carbon monoxide (CO), and other man-made compounds are present in smaller concentrations and are also part of the atmospheric carbon cycle.
The evolution of photosynthesis refers to the origin and subsequent evolution of photosynthesis, the process by which light energy is used to assemble sugars from carbon dioxide and a hydrogen and electron source such as water. The process of photosynthesis was discovered by Jan Ingenhousz, a Dutch-born British physician and scientist, first publishing about it in 1779.
Minze Stuiver was a Dutch geochemist who was at the forefront of geoscience research from the 1960s until his retirement in 1998. He helped transform radiocarbon dating from a simple tool for archaeology and geology to a precise technique with applications in solar physics, oceanography, geochemistry, and carbon dynamics. Minze Stuiver's research encompassed the use of radiocarbon (14C) to understand solar cycles and radiocarbon production, ocean circulation, lake carbon dynamics and archaeology as well as the use of stable isotopes to document past climate changes.
The variation in the 14
C/12
C ratio in different parts of the carbon exchange reservoir means that a straightforward calculation of the age of a sample based on the amount of 14
C it contains will often give an incorrect result. There are several other possible sources of error that need to be considered. The errors are of four general types:
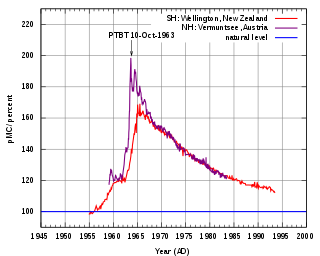
The bomb pulse is the sudden increase of carbon-14 (14C) in the Earth's atmosphere due to the hundreds of aboveground nuclear bombs tests that started in 1945 and intensified after 1950 until 1963, when the Limited Test Ban Treaty was signed by the United States, the Soviet Union and the United Kingdom. These hundreds of blasts were followed by a doubling of the relative concentration of 14C in the atmosphere. The reason for the term “relative concentration”, is because the measurements of 14C levels by mass spectrometers are most accurately made by comparison to another carbon isotope, often the common isotope 12C. Isotope abundance ratios are not only more easily measured, they are what 14C carbon daters want, since it is the fraction of carbon in a sample that is 14C, not the absolute concentration, that is of interest in dating measurements. The figure shows how the fraction of carbon in the atmosphere that is 14C, of order only a part per trillion, has changed over the past several decades following the bomb tests. Because 12C concentration has increased by about 30% over the past fifty years, the fact that “pMC”, measuring the isotope ratio, has returned (almost) to its 1955 value, means that 14C concentration in the atmosphere remains some 30% higher than it once was. Carbon-14, the radioisotope of carbon, is naturally developed in trace amounts in the atmosphere and it can be detected in all living organisms. Carbon of all types is continually used to form the molecules of the cells of organisms. Doubling of the concentration of 14C in the atmosphere is reflected in the tissues and cells of all organisms that lived around the period of nuclear testing. This property has many applications in the fields of biology and forensics.

Photosynthesis converts carbon dioxide to carbohydrates via several metabolic pathways that provide energy to an organism and preferentially react with certain stable isotopes of carbon. The selective enrichment of one stable isotope over another creates distinct isotopic fractionations that can be measured and correlated among oxygenic phototrophs. The degree of carbon isotope fractionation is influenced by several factors, including the metabolism, anatomy, growth rate, and environmental conditions of the organism. Understanding these variations in carbon fractionation across species is useful for biogeochemical studies, including the reconstruction of paleoecology, plant evolution, and the characterization of food chains.

The kinetic isotope effect (KIE) of ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) is the isotopic fractionation associated solely with the step in the Calvin-Benson cycle where a molecule of carbon dioxide is attached to the 5-carbon sugar ribulose-1,5-bisphosphate (RuBP) to produce two 3-carbon sugars called 3-phosphoglycerate. This chemical reaction is catalyzed by the enzyme RuBisCO, and this enzyme-catalyzed reaction creates the primary kinetic isotope effect of photosynthesis. It is also largely responsible for the isotopic compositions of photosynthetic organisms and the heterotrophs that eat them. Understanding the intrinsic KIE of RuBisCO is of interest to earth scientists, botanists, and ecologists because this isotopic biosignature can be used to reconstruct the evolution of photosynthesis and the rise of oxygen in the geologic record, reconstruct past evolutionary relationships and environmental conditions, and infer plant relationships and productivity in modern environments.
Isotope analysis has many applications in archaeology, from dating sites and artefacts, determination of past diets and migration patterns and for environmental reconstruction.
References
- ↑ Tans, P.P.; de Jong, A.F.M.; Mook, W. G. (30 August 1979). "Natural atmospheric 14C variation and the Suess effect". Nature . 280 (5725): 826–828. Bibcode:1979Natur.280..826T. doi:10.1038/280826a0. S2CID 4323299.
- ↑ "CARD: What is the Suess effect?". Canadian Archaeological Radioactive Database. Archived from the original on 2007-09-29. Retrieved 2007-10-19.
- ↑ Keeling, C. D. (1979). "The Suess effect: 13Carbon-14Carbon interrelations". Environment International. 2 (4–6): 229–300. Bibcode:1979EnInt...2..229K. doi:10.1016/0160-4120(79)90005-9.
- ↑ Schwartz, S. E.; Hua, Q.; Andrews, D. E.; Keeling, R. F.; Lehman, S. J.; Turnbull, J. C.; Reimer, P, J.; Miller, J. B.; Meijer, H. A. J. (2024). "Discussion: Presentation of Atmospheric 14CO2 Data". Radiocarbon. xx (xx): 1–14. doi:10.1017/RDC.2024.27.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Farquhar, G. D.; Ehleringer, J. R.; Hubick, K. T. (1989). "Carbon Isotope Discrimination and Photosynthesis". Annu. Rev. Plant Physiol. Plant Mol. Biol. 40: 503–537. doi:10.1146/annurev.pp.40.060189.002443.
- ↑ Bozhinova, D.; van der Molen, M. K.; van der Velde, I. R.; Krol, M. C.; van der Laan, S.; Meijer, H. A. J.; Peters, W. (17 July 2014). "Simulating the integrated summertime Δ14CO2 signature from anthropogenic emissions over Western Europe". Atmos. Chem. Phys. 14 (14): 7273–7290. doi: 10.5194/acp-14-7273-2014 .