
A brain tumor occurs when abnormal cells form within the brain. There are two main types of tumors: malignant (cancerous) tumors and benign (non-cancerous) tumors. These can be further classified as primary tumors, which start within the brain, and secondary tumors, which most commonly have spread from tumors located outside the brain, known as brain metastasis tumors. All types of brain tumors may produce symptoms that vary depending on the size of the tumor and the part of the brain that is involved. Where symptoms exist, they may include headaches, seizures, problems with vision, vomiting and mental changes. Other symptoms may include difficulty walking, speaking, with sensations, or unconsciousness.

Ovarian cancer is a cancerous tumor of an ovary. It may originate from the ovary itself or more commonly from communicating nearby structures such as fallopian tubes or the inner lining of the abdomen. The ovary is made up of three different cell types including epithelial cells, germ cells, and stromal cells. When these cells become abnormal, they have the ability to divide and form tumors. These cells can also invade or spread to other parts of the body. When this process begins, there may be no or only vague symptoms. Symptoms become more noticeable as the cancer progresses. These symptoms may include bloating, vaginal bleeding, pelvic pain, abdominal swelling, constipation, and loss of appetite, among others. Common areas to which the cancer may spread include the lining of the abdomen, lymph nodes, lungs, and liver.

Small-cell carcinoma is a type of highly malignant cancer that most commonly arises within the lung, although it can occasionally arise in other body sites, such as the cervix, prostate, and gastrointestinal tract. Compared to non-small cell carcinoma, small cell carcinoma is more aggressive, with a shorter doubling time, higher growth fraction, and earlier development of metastases.

Angiosarcoma is a rare and aggressive cancer that starts in the endothelial cells that line the walls of blood vessels or lymphatic vessels. Since they are made from vascular lining, they can appear anywhere and at any age, but older people are more commonly affected, and the skin is the most affected area, with approximately 60% of cases being cutaneous (skin). Specifically, the scalp makes up ~50% of angiosarcoma cases, but this is still <0.1% of all head and neck tumors. Since angiosarcoma is an umbrella term for many types of tumor that vary greatly in origin and location, many symptoms may occur, from completely asymptomatic to non-specific symptoms like skin lesions, ulceration, shortness of breath and abdominal pain. Multiple-organ involvement at time of diagnosis is common and makes it difficult to ascertain origin and how to treat it.
Clinical endpoints or clinical outcomes are outcome measures referring to occurrence of disease, symptom, sign or laboratory abnormality constituting a target outcome in clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a humane (clinical) endpoint.

Invasive carcinoma of no special type, invasive breast carcinoma of no special type (IBC-NST), invasive ductal carcinoma (IDC), infiltrating ductal carcinoma (IDC) or invasive ductal carcinoma, not otherwise specified (NOS) is a disease. For international audiences this article will use "invasive carcinoma NST" because it is the preferred term of the World Health Organization (WHO).

Sunitinib, sold under the brand name Sutent, is an anti-cancer medication. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) in January 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.
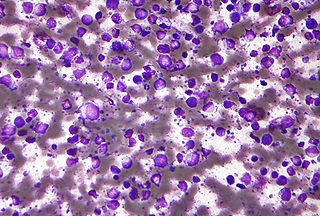
Diffuse large B-cell lymphoma (DLBCL) is a cancer of B cells, a type of lymphocyte that is responsible for producing antibodies. It is the most common form of non-Hodgkin lymphoma among adults, with an annual incidence of 7–8 cases per 100,000 people per year in the US and UK. This cancer occurs primarily in older individuals, with a median age of diagnosis at ~70 years, although it can occur in young adults and, in rare cases, children. DLBCL can arise in virtually any part of the body and, depending on various factors, is often a very aggressive malignancy. The first sign of this illness is typically the observation of a rapidly growing mass or tissue infiltration that is sometimes associated with systemic B symptoms, e.g. fever, weight loss, and night sweats.
Response evaluation criteria in solid tumors (RECIST) is a set of published rules that define when tumors in cancer patients improve ("respond"), stay the same ("stabilize"), or worsen ("progress") during treatment. The criteria were published in February 2000 by an international collaboration including the European Organisation for Research and Treatment of Cancer (EORTC), National Cancer Institute of the United States, and the National Cancer Institute of Canada Clinical Trials Group. Today, the majority of clinical trials evaluating cancer treatments for objective response in solid tumors use RECIST. These criteria were developed and published in February 2000, and subsequently updated in 2009.
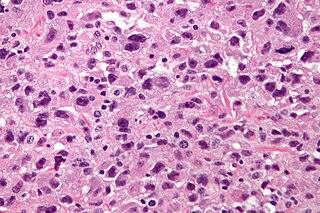
Undifferentiated pleomorphic sarcoma (UPS), also termed pleomorphic myofibrosarcoma, high-grade myofibroblastic sarcoma, and high-grade myofibrosarcoma, is characterized by the World Health Organization (WHO) as a rare, poorly differentiated neoplasm. WHO classified it as one of the undifferentiated/unclassified sarcomas in the category of tumors of uncertain differentiation. Sarcomas are cancers derived mesenchymal stem cells that typically develop in bone, muscle, fat, blood vessels, lymphatic vessels, tendons, and ligaments. More than 70 sarcoma subtypes have been described. The UPS subtype of these sarcomas consists of tumor cells that are poorly differentiated and may appear as spindle-shaped cells, histiocytes, and giant cells. UPS is considered a diagnosis that defies formal sub-classification after thorough histologic, immunohistochemical, and ultrastructural examinations fail to identify the type of cells involved.
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis is the assessment that a particular medical condition is present while an indication is a reason for use. The opposite of an indication is a contraindication, a reason to withhold a certain medical treatment because the risks of treatment clearly outweigh the benefits.
Triple-negative breast cancer (TNBC) is any breast cancer that either lacks or shows low levels of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) overexpression and/or gene amplification. Triple-negative is sometimes used as a surrogate term for basal-like.
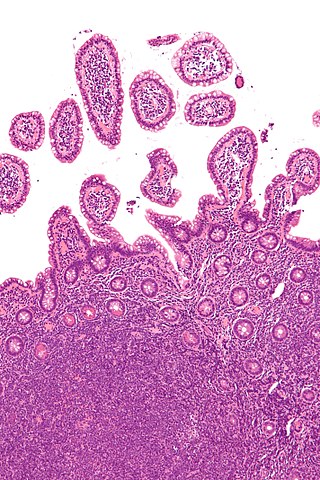
Mantle cell lymphoma (MCL) is a type of non-Hodgkin's lymphoma, comprising about 6% of cases. It is named for the mantle zone of the lymph nodes where it develops. The term 'mantle cell lymphoma' was first adopted by Raffeld and Jaffe in 1991.
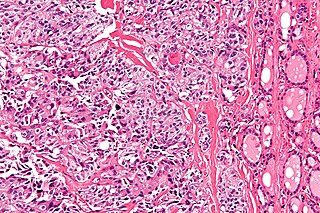
Medullary thyroid cancer is a form of thyroid carcinoma which originates from the parafollicular cells, which produce the hormone calcitonin. Medullary tumors are the third most common of all thyroid cancers and together make up about 3% of all thyroid cancer cases. MTC was first characterized in 1959.

Leptomeningeal cancer is a rare complication of cancer in which the disease spreads from the original tumor site to the meninges surrounding the brain and spinal cord. This leads to an inflammatory response, hence the alternative names neoplastic meningitis (NM), malignant meningitis, or carcinomatous meningitis. The term leptomeningeal describes the thin meninges, the arachnoid and the pia mater, between which the cerebrospinal fluid is located. The disorder was originally reported by Eberth in 1870. It is also known as leptomeningeal carcinomatosis, leptomeningeal disease (LMD), leptomeningeal metastasis, meningeal metastasis and meningeal carcinomatosis.
Progression-free survival (PFS) is "the length of time during and after the treatment of a disease, such as cancer, that a patient lives with the disease but it does not get worse". In oncology, PFS usually refers to situations in which a tumor is present, as demonstrated by laboratory testing, radiologic testing, or clinically. Similarly, "disease-free survival" is the length of time after patients have received treatment and have no detectable disease.

A brain metastasis is a cancer that has metastasized (spread) to the brain from another location in the body and is therefore considered a secondary brain tumor. The metastasis typically shares a cancer cell type with the original site of the cancer. Metastasis is the most common cause of brain cancer, as primary tumors that originate in the brain are less common. The most common sites of primary cancer which metastasize to the brain are lung, breast, colon, kidney, and skin cancer. Brain metastases can occur months or even years after the original or primary cancer is treated. Brain metastases have a poor prognosis for cure, but modern treatments allow patients to live months and sometimes years after the diagnosis.

Trastuzumab emtansine, sold under the brand name Kadcyla, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the cytotoxic agent DM1. Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death. Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells. The conjugate is abbreviated T-DM1.

Durvalumab, sold under the brand name Imfinzi, is an FDA-approved immunotherapy for cancer, developed by Medimmune/AstraZeneca. It is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that blocks the interaction of programmed cell death ligand 1 (PD-L1) with the PD-1 (CD279).

Lurbinectedin, sold under the brand name Zepzelca, is a medication used for the treatment of small cell lung cancer.














