Related Research Articles
The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part of the 6d transition series. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear weapons reprocessing. The storage and disposal of radioactive waste is regulated by government agencies in order to protect human health and the environment.
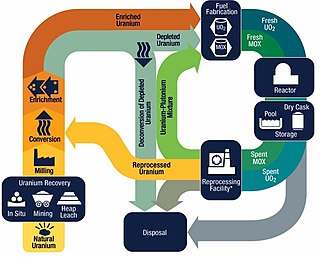
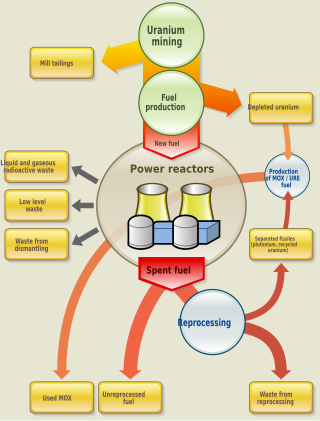
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.

Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactors. The reprocessed uranium, also known as the spent fuel material, can in principle also be re-used as fuel, but that is only economical when uranium supply is low and prices are high. Nuclear reprocessing may extend beyond fuel and include the reprocessing of other nuclear reactor material, such as Zircaloy cladding.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the light-water reactors that predominate nuclear power generation.

Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
Vitrification is the full or partial transformation of a substance into a glass, that is to say, a non-crystalline or amorphous solid. Glasses differ from liquids structurally and glasses possess a higher degree of connectivity with the same Hausdorff dimensionality of bonds as crystals: dimH = 3. In the production of ceramics, vitrification is responsible for their impermeability to water.

The Savannah River Site (SRS) is a U.S. Department of Energy (DOE) reservation in the United States, located in the state of South Carolina on land in Aiken, Allendale, and Barnwell counties adjacent to the Savannah River. It lies 25 miles (40 km) southeast of Augusta, Georgia. The site was built during the 1950s to refine nuclear materials for deployment in nuclear weapons. It covers 310 square miles (800 km2) and employs more than 10,000 people.

Hot isostatic pressing (HIP) is a manufacturing process, used to reduce the porosity of metals and increase the density of many ceramic materials. This improves the material's mechanical properties and workability.

The integral fast reactor (IFR), originally the advancedliquid-metal reactor (ALMR), is a design for a nuclear reactor using fast neutrons and no neutron moderator. IFRs can breed more fuel and are distinguished by a nuclear fuel cycle that uses reprocessing via electrorefining at the reactor site.
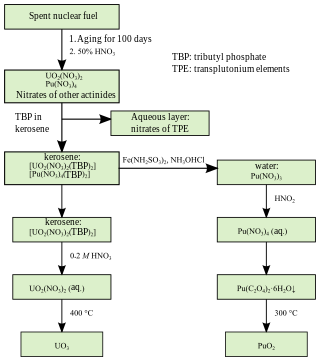
PUREX is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid–liquid extraction ion-exchange.
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other nuclear devices to generate energy.

The La Hague site is a nuclear fuel reprocessing plant at La Hague on the Cotentin Peninsula in northern France, with the Manche storage centre bordering on it. Operated by Orano, formerly AREVA, and prior to that COGEMA, La Hague has nearly half of the world's light water reactor spent nuclear fuel reprocessing capacity. It has been in operation since 1976, and has a capacity of about 1,700 tonnes per year. It extracts plutonium which is then recycled into MOX fuel at the Marcoule site.

High-level waste (HLW) is a type of nuclear waste created by the reprocessing of spent nuclear fuel. It exists in two main forms:
DUCRETE is a high density concrete alternative investigated for use in construction of casks for storage of radioactive waste. It is a composite material containing depleted uranium dioxide aggregate instead of conventional gravel, with a Portland cement binder.

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor. It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and, depending on its point along the nuclear fuel cycle, it will have different isotopic constituents than when it started.

The actinide series is a group of chemical elements with atomic numbers ranging from 89 to 102, including notable elements such as uranium and plutonium. The nuclides thorium-232, uranium-235, and uranium-238 occur primordially, while trace quantities of actinium, protactinium, neptunium, and plutonium exist as a result of radioactive decay and neutron capture of uranium. These elements are far more radioactive than the naturally occurring thorium and uranium, and thus have much shorter half-lives. Elements with atomic numbers greater than 94 do not exist naturally on Earth, and must be produced in a nuclear reactor. However, certain isotopes of elements up to californium still have practical applications which take advantage of their radioactive properties.

High-level radioactive waste management addresses the handling of radioactive materials generated from nuclear power production and nuclear weapons manufacture. Radioactive waste contains both short-lived and long-lived radionuclides, as well as non-radioactive nuclides. In 2002, the United States stored approximately 47,000 tonnes of high-level radioactive waste.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
The advanced reprocessing of spent nuclear fuel is a potential key to achieve a sustainable nuclear fuel cycle and to tackle the heavy burden of nuclear waste management. In particular, the development of such advanced reprocessing systems may save natural resources, reduce waste inventory and enhance the public acceptance of nuclear energy. This strategy relies on the recycling of major actinides and the transmutation of minor actinides in appropriate reactors. In order to fulfill this objective, selective extracting agents need to be designed and developed by investigating their complexation mechanism.
References
- 1 2 "Synroc – World Nuclear Association". www.world-nuclear.org.
- ↑ W. E. Lee, M.I. Ojovan, C.M. Jantzen. Radioactive waste management and contaminated site clean-up: Processes, technologies and international experience, Woodhead, Cambridge, 924 p. (2013). www.woodheadpublishing.com/9780857094353
- ↑ B.E. Burakov, M.I Ojovan, W.E. Lee. Crystalline Materials for Actinide Immobilisation, Imperial College Press, London, 198 pp. (2010). "Crystalline Materials for Actinide Immobilisation". Archived from the original on 2012-03-09. Retrieved 2010-10-16.
- ↑ Ron Cameron, Chief of Operations, ANSTO. The (half)-life of waste. "Ask and Expert, Nuclear Power". Australian Broadcasting Corporation . 27 October 2005.
- ↑ E.R Vance, D.J Gregg and D.T Chavara, ANSTO. "Past and present Applications of Synroc" (PDF).
- ↑ US Department of Energy (January 4, 2010), Federal Register (excerpt) (PDF), vol. 75/1, pp. 137–140, retrieved May 5, 2010
- ↑ "ANSTO Inc HIP demonstration contract award" (PDF) (Press release). ANSTO. April 1, 2008. Retrieved May 5, 2010.