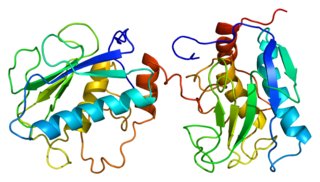Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily.

72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the MMP2 gene. The MMP2 gene is located on chromosome 16 at position 12.2.

Matrix metalloproteinase-14 is an enzyme that in humans is encoded by the MMP14 gene.
Interstitial collagenase, also known as fibroblast collagenase and matrix metalloproteinase-1 (MMP-1), is an enzyme that in humans is encoded by the MMP1 gene. The gene is part of a cluster of MMP genes which localize to chromosome 11q22.3. MMP-1 was the first vertebrate collagenase both purified to homogeneity as a protein, and cloned as a cDNA. MMP-1 has an estimated molecular mass of 54 kDa.
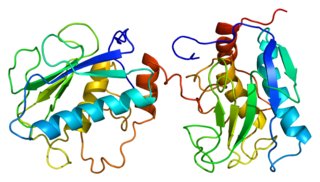
Stromelysin-1 also known as matrix metalloproteinase-3 (MMP-3) is an enzyme that in humans is encoded by the MMP3 gene. The MMP3 gene is part of a cluster of MMP genes which localize to chromosome 11q22.3. MMP-3 has an estimated molecular weight of 54 kDa.
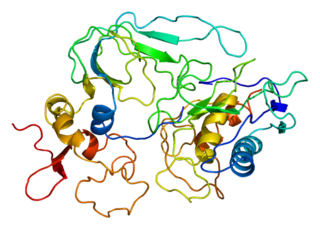
Tissue inhibitor of metalloproteinases 2 (TIMP2) is a gene and a corresponding protein. The gene is a member of the TIMP gene family. The protein is thought to be a metastasis suppressor.

Matrilysin also known as matrix metalloproteinase-7 (MMP-7), pump-1 protease (PUMP-1), or uterine metalloproteinase is an enzyme in humans that is encoded by the MMP7 gene. The enzyme has also been known as matrin, putative metalloproteinase-1, matrix metalloproteinase pump 1, PUMP-1 proteinase, PUMP, metalloproteinase pump-1, putative metalloproteinase, MMP). Human MMP-7 has a molecular weight around 30 kDa.

Kallikrein-2 is a protein that in humans is encoded by the KLK2 gene, and is particularly associated with prostatic tissue.

Collagenase 3 is an enzyme that in humans is encoded by the MMP13 gene. It is a member of the matrix metalloproteinase (MMP) family. Like most MMPs, it is secreted as an inactive pro-form. MMP-13 has an predicted molecular weight around 54 kDa. It is activated once the pro-domain is cleaved, leaving an active enzyme composed of the catalytic domain and the hemopexin-like domain PDB: 1PEX. Although the actual mechanism has not been described, the hemopexin domain participates in collagen degradation, the catalytic domain alone being particularly inefficient in collagen degradation. During embryonic development, MMP-13 is expressed in the skeleton as required for restructuring the collagen matrix for bone mineralization. In pathological situations it is highly overexpressed; this occurs in human carcinomas, rheumatoid arthritis and osteoarthritis.
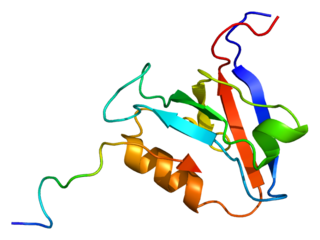
Tyrosine-protein phosphatase non-receptor type 13 is an enzyme that in humans is encoded by the PTPN13 gene.

Matrix metalloproteinase-12 (MMP-12) also known as macrophage metalloelastase (MME) or macrophage elastase (ME) is an enzyme that in humans is encoded by the MMP12 gene.

Matrix metalloproteinase-26 also known as matrilysin-2 and endometase is an enzyme that in humans is encoded by the MMP26 gene.

Matrix metalloproteinase-19 (MMP-19) also known as matrix metalloproteinase RASI is an enzyme that in humans is encoded by the MMP19 gene.

LIM/homeobox protein Lhx3 is a protein that in humans is encoded by the LHX3 gene.

Matrix metalloproteinase-17 (MMP-17) also known as membrane-type matrix metalloproteinase 4 is an enzyme that in humans is encoded by the MMP17 gene.
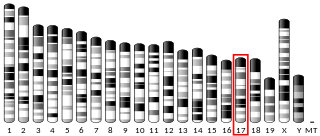
Matrix metalloproteinase-25 is an enzyme that in humans is encoded by the MMP25 gene.

Matrix metallopeptidase 27 also known as MMP-27 is an enzyme which in humans is encoded by the MMP27 gene.

Matrix metalloproteinase 15 also known as MMP15 is an enzyme that in humans is encoded by the MMP15 gene.
Angiogenesis is the process of forming new blood vessels from existing blood vessels, formed in vasculogenesis. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAM), a disintegrin and metalloproteinase domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.
The hemopexin family is a family of evolutionarily related proteins. Hemopexin-like repeats occur in vitronectin and some matrix metalloproteinases family (matrixins). The HX repeats of some matrixins bind tissue inhibitor of metallopeptidases (TIMPs).