
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

Paclitaxel, sold under the brand name Taxol among others, is a chemotherapy medication used to treat ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma, cervical cancer, and pancreatic cancer. It is administered by intravenous injection. There is also an albumin-bound formulation.

In cell biology, the spindle apparatus is the cytoskeletal structure of eukaryotic cells that forms during cell division to separate sister chromatids between daughter cells. It is referred to as the mitotic spindle during mitosis, a process that produces genetically identical daughter cells, or the meiotic spindle during meiosis, a process that produces gametes with half the number of chromosomes of the parent cell.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. It was discovered and named by Hideo Mōri in 1968. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.

Docetaxel, sold under the brand name Taxotere among others, is a chemotherapy medication used to treat a number of types of cancer. This includes breast cancer, head and neck cancer, stomach cancer, prostate cancer and non-small-cell lung cancer. It may be used by itself or along with other chemotherapy medication. It is given by slow injection into a vein.
A spindle poison, also known as a spindle toxin, is a poison that disrupts cell division by affecting the protein threads that connect the centromere regions of chromosomes, known as spindles. Spindle poisons effectively cease the production of new cells by interrupting the mitosis phase of cell division at the spindle assembly checkpoint (SAC). However, as numerous and varied as they are, spindle poisons are not yet 100% effective at ending the formation of tumors (neoplasms). Although not 100% effective, substantive therapeutic efficacy has been found in these types of chemotherapeutic treatments. The mitotic spindle is composed of microtubules that aid, along with regulatory proteins, each other in the activity of appropriately segregating replicated chromosomes. Certain compounds affecting the mitotic spindle have proven highly effective against solid tumors and hematological malignancies.

Taxanes are a class of diterpenes. They were originally identified from plants of the genus Taxus (yews), and feature a taxadiene core. Paclitaxel (Taxol) and docetaxel (Taxotere) are widely used as chemotherapy agents. Cabazitaxel was FDA approved to treat hormone-refractory prostate cancer.

Epothilones are a class of potential cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials, epothilones have better efficacy and milder adverse effects than taxanes.

Combretastatin is a dihydrostilbenoid found in Combretum afrum.

Cryptophycins are a family of macrolide molecules that are potent cytotoxins and have been studied for potential antiproliferative properties useful in developing chemotherapy. They are members of the depsipeptide family.

Ixabepilone is a pharmaceutical drug developed by Bristol-Myers Squibb as a chemotherapeutic medication for cancer.

A mitotic inhibitor, microtubule inhibitor, or tubulin inhibitor, is a drug that inhibits mitosis, or cell division, and is used in treating cancer, gout, and nail fungus. These drugs disrupt microtubules, which are structures that pull the chromosomes apart when a cell divides. Mitotic inhibitors are used in cancer treatment, because cancer cells are able to grow through continuous division that eventually spread through the body (metastasize). Thus, cancer cells are more sensitive to inhibition of mitosis than normal cells. Mitotic inhibitors are also used in cytogenetics, where they stop cell division at a stage where chromosomes can be easily examined.

Tubulin beta-3 chain, Class III β-tubulin, βIII-tubulin (β3-tubulin) or β-tubulin III, is a microtubule element of the tubulin family found almost exclusively in neurons, and in testis cells. In humans, it is encoded by the TUBB3 gene.

Eribulin, sold under the brand name Halaven among others, is an anti-cancer medication used to treat breast cancer and liposarcoma.

Paclitaxel trevatide is an experimental chemotherapy drug that is under development by Angiochem Inc, a Canadian biotech company. Phase II clinical trials have completed for several indications, and the company is preparing for phase III trials.

Abeotaxanes are a class of taxoid molecules with a core 5/7/6 type ring structure. This structure varies from the 6/8/6 or 6/10/6-membered core ring found in conventional taxoids such as paclitaxel or docetaxel. The core carbon skeleton of a normal natural product taxane has a 6-membered A ring, 8-membered B ring and a 6-membered C ring, combined with conventional side chains. It is the side chains that provide most of the activity for regular taxanes. Abeotaxanes are compounds containing 3 altered ring structures, where ring A is 5 members, ring B is 7 members and ring C is 6 members, combined with conventional side chains.
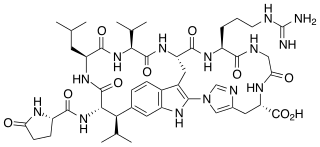
Moroidin is a biologically active compound found in the plants Dendrocnide moroides and Celosia argentea. It is a peptide composed of eight amino acids, with unusual leucine-tryptophan and tryptophan-histidine cross-links that form its two rings. Moroidin has been shown to be at least one of several bioactive compounds responsible for the painful sting of the Dendrocnide moroides plant. It also has demonstrated anti-mitotic properties, specifically by inhibition of tubulin polymerization. Anti-mitotic activity gives moroidin potential as a chemotherapy drug, and this property combined with its unusual chemical structure has made it a target for organic synthesis.

2-Methoxyestradiol disulfamate is a synthetic, oral active anti-cancer medication which was previously under development for potential clinical use. It has improved potency, low metabolism, and good pharmacokinetic properties relative to 2-methoxyestradiol (2-MeO-E2). It is also a potent inhibitor of steroid sulfatase, the enzyme that catalyzes the desulfation of steroids such as estrone sulfate and dehydroepiandrosterone sulfate (DHEA-S).
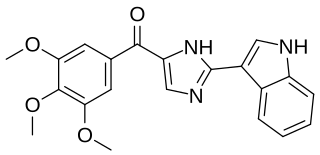
Sabizabulin is an investigational new drug that is being evaluated for the treatment of castration-resistant prostate cancer and in SARS-CoV-2 (COVID-19) infections. It is a tubulin polymerization inhibitor.

Sagopilone is a fully synthetic macrolide of the epothilone family with the molecular formula C30H41NO6S. The mechanism of action is similar to taxanes, as they bind to the microtubule and prohibit cell division. These toxic properties and its possibility to cross the blood-brain barrier makes it a promising cancer medication.


















