
Staphylococcus aureus is a gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe, meaning that it can grow without oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA). The bacterium is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.

Vancomycin is a glycopeptide antibiotic medication used to treat certain bacterial infections. It is administered intravenously to treat complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also taken orally to treat severe Clostridioides difficile infection. Taken orally, it is poorly absorbed.

Methicillin-resistant Staphylococcus aureus (MRSA) is a group of gram-positive bacteria that are genetically distinct from other strains of Staphylococcus aureus. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths worldwide attributable to antimicrobial resistance in 2019.

Linezolid is an antibiotic used for the treatment of infections caused by Gram-positive bacteria that are resistant to other antibiotics. Linezolid is active against most Gram-positive bacteria that cause disease, including streptococci, vancomycin-resistant enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA). The main uses are infections of the skin and pneumonia although it may be used for a variety of other infections including drug-resistant tuberculosis. It is used either by injection into a vein or by mouth.

Clindamycin is a lincosamide antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media, and endocarditis. It can also be used to treat acne, and some cases of methicillin-resistant Staphylococcus aureus (MRSA). In combination with quinine, it can be used to treat malaria. It is available by mouth, by injection into a vein, and as a cream or a gel to be applied to the skin or in the vagina.

Fusidic acid, sold under the brand name Fucidin among others, is a steroid antibiotic that is often used topically in creams or ointments and eyedrops but may also be given systemically as tablets or injections. As of October 2008, the global problem of advancing antimicrobial resistance has led to a renewed interest in its use.

Vancomycin-resistant Staphylococcus aureus (VRSA) are strains of Staphylococcus aureus that have acquired resistance to the glycopeptide antibiotic vancomycin. Bacteria can acquire resistant genes either by random mutation or through the transfer of DNA from one bacterium to another. Resistance genes interfere with the normal antibiotic function and allow bacteria to grow in the presence of the antibiotic. Resistance in VRSA is conferred by the plasmid-mediated vanA gene and operon. Although VRSA infections are uncommon, VRSA is often resistant to other types of antibiotics and a potential threat to public health because treatment options are limited. VRSA is resistant to many of the standard drugs used to treat S. aureus infections. Furthermore, resistance can be transferred from one bacterium to another.

Glycopeptide antibiotics are a class of drugs of microbial origin that are composed of glycosylated cyclic or polycyclic nonribosomal peptides. Significant glycopeptide antibiotics include the anti-infective antibiotics vancomycin, teicoplanin, telavancin, ramoplanin and decaplanin, corbomycin, complestatin and the antitumor antibiotic bleomycin. Vancomycin is used if infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.

Tigecycline, sold under the brand name Tygacil, is a tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline class drug that is administered intravenously. It was developed in response to the growing rate of antibiotic resistant bacteria such as Staphylococcus aureus, Acinetobacter baumannii, and E. coli. As a tetracycline derivative antibiotic, its structural modifications has expanded its therapeutic activity to include Gram-positive and Gram-negative organisms, including those of multi-drug resistance.

Cefotaxime is an antibiotic used to treat several bacterial infections in humans, other animals, and plant tissue culture. Specifically in humans it is used to treat joint infections, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, sepsis, gonorrhea, and cellulitis. It is given either by injection into a vein or muscle.
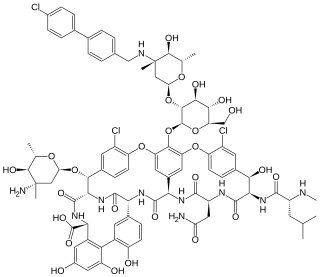
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.

Ceftobiprole, sold under the brand name Zevtera among others, is a fifth-generation cephalosporin antibacterial used for the treatment of hospital-acquired pneumonia and community-acquired pneumonia. It is marketed by Basilea Pharmaceutica under the brand names Zevtera and Mabelio. Like other cephalosporins, ceftobiprole exerts its antibacterial activity by binding to important penicillin-binding proteins and inhibiting their transpeptidase activity which is essential for the synthesis of bacterial cell walls. Ceftobiprole has high affinity for penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus strains and retains its activity against strains that express divergent mecA gene homologues. Ceftobiprole also binds to penicillin-binding protein 2b in Streptococcus pneumoniae (penicillin-intermediate), to penicillin-binding protein 2x in Streptococcus pneumoniae (penicillin-resistant), and to penicillin-binding protein 5 in Enterococcus faecalis.

Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatments available to people infected with methicillin-resistant Staphylococcus aureus (MRSA).

Telavancin is a bactericidal lipoglycopeptide for use in MRSA or other Gram-positive infections. Telavancin is a semi-synthetic derivative of vancomycin.

Iclaprim is an antibiotic drug candidate that is active against Gram positive organisms. It is administered intravenously.

Tedizolid, sold under the brand name Sivextro is an oxazolidinone-class antibiotic. Tedizolid phosphate is a phosphate ester prodrug of the active compound tedizolid. It was developed by Cubist Pharmaceuticals, following acquisition of Trius Therapeutics, and is marketed for the treatment of acute bacterial skin and skin structure infections.

Ceftaroline fosamil (INN), brand name Teflaro in the US and Zinforo in Europe, is a cephalosporin antibiotic with anti-MRSA activity. Ceftaroline fosamil is a prodrug of ceftaroline. It is active against methicillin-resistant Staphylococcus aureus (MRSA) and other Gram-positive bacteria. It retains some activity of later-generation cephalosporins having broad-spectrum activity against Gram-negative bacteria, but its effectiveness is relatively much weaker. It is currently being investigated for community-acquired pneumonia and complicated skin and skin structure infection.

Lipoglycopeptides are a class of antibiotic that have lipophilic side-chains linked to glycopeptides. The class includes oritavancin, telavancin and dalbavancin.

Anthracimycin is a polyketide antibiotic discovered in 2013. Anthracimycin is derived from marine actinobacteria. In preliminary laboratory research, it has shown activity against Bacillus anthracis, the bacteria that causes anthrax, and against methicillin-resistant Staphylococcus aureus (MRSA).
Kerry L. LaPlante is an American pharmacist, academic and researcher. She is the Dean at the University of Rhode Island College of Pharmacy. She is a Professor of Pharmacy and former department Chair of the Department of Pharmacy Practice at the University of Rhode Island, an adjunct professor of medicine at Brown University, an Infectious Diseases Pharmacotherapy Specialist, and the Director of the Rhode Island Infectious Diseases Fellowship and Research Programs at the Veterans Affairs Medical Center in Providence, Rhode Island.

















