Related Research Articles

Haematopoiesis is the formation of blood cellular components. All cellular blood components are derived from haematopoietic stem cells. In a healthy adult human, roughly ten billion to a hundred billion new blood cells are produced per day, in order to maintain steady state levels in the peripheral circulation.

Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm.

CD34 is a transmembrane phosphoglycoprotein protein encoded by the CD34 gene in humans, mice, rats and other species.
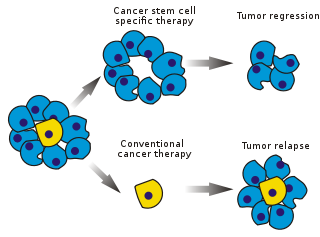
Cancer stem cells (CSCs) are cancer cells that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are therefore tumorigenic (tumor-forming), perhaps in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are hypothesized to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for patients with metastatic disease.
In immunology, central tolerance is the process of eliminating any developing T or B lymphocytes that are autoreactive, i.e. reactive to the body itself. Through elimination of autoreactive lymphocytes, tolerance ensures that the immune system does not attack self peptides. Lymphocyte maturation occurs in primary lymphoid organs such as the bone marrow and the thymus. In mammals, B cells mature in the bone marrow and T cells mature in the thymus.
Hemangioblasts are the multipotent precursor cells that can differentiate into both hematopoietic and endothelial cells. In the mouse embryo, the emergence of blood islands in the yolk sac at embryonic day 7 marks the onset of hematopoiesis. From these blood islands, the hematopoietic cells and vasculature are formed shortly after. Hemangioblasts are the progenitors that form the blood islands. To date, the hemangioblast has been identified in human, mouse and zebrafish embryos.

Thy-1 or CD90 is a 25–37 kDa heavily N-glycosylated, glycophosphatidylinositol (GPI) anchored conserved cell surface protein with a single V-like immunoglobulin domain, originally discovered as a thymocyte antigen. Thy-1 can be used as a marker for a variety of stem cells and for the axonal processes of mature neurons. Structural study of Thy-1 led to the foundation of the Immunoglobulin superfamily, of which it is the smallest member, and led to some of the initial biochemical description and characterization of a vertebrate GPI anchor and also the first demonstration of tissue specific differential glycosylation.
Lymphopoiesis (lĭm'fō-poi-ē'sĭs) is the generation of lymphocytes, one of the five types of white blood cells (WBCs). It is more formally known as lymphoid hematopoiesis.

Proto-oncogene c-KIT is the gene encoding the receptor tyrosine kinase protein known as tyrosine-protein kinase KIT, CD117 or mast/stem cell growth factor receptor (SCFR). Multiple transcript variants encoding different isoforms have been found for this gene. KIT was first described by the German biochemist Axel Ullrich in 1987 as the cellular homolog of the feline sarcoma viral oncogene v-kit.

Integrin α4β1 is an integrin dimer. It is composed of CD49d and CD29. The alpha 4 subunit is 155 kDa, and the beta 1 subunit is 150 kDa.
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, macrophages, and certain T cells. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.
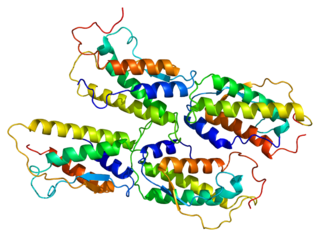
Stem cell factor is a cytokine that binds to the c-KIT receptor (CD117). SCF can exist both as a transmembrane protein and a soluble protein. This cytokine plays an important role in hematopoiesis, spermatogenesis, and melanogenesis.

Fms-related tyrosine kinase 3 ligand (FLT3LG) is a protein which in humans is encoded by the FLT3LG gene.
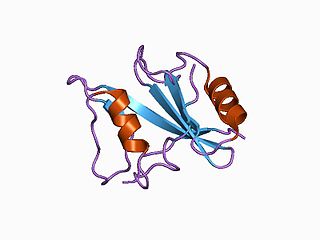
Signaling lymphocytic activation molecule 1 is a protein that in humans is encoded by the SLAMF1 gene. Recently SLAMF1 has also been designated CD150.

CD109 is a human gene.
Stem cell markers are genes and their protein products used by scientists to isolate and identify stem cells. Stem cells can also be identified by functional assays. Below is a list of genes/protein products that can be used to identify various types of stem cells, or functional assays that do the same. The initial version of the list below was obtained by mining the PubMed database as described in
KSL cells in cell biology are an early form of mouse/murine hematopoietic stem cells. Characteristics are Kit (+), Sca-1 (+) and Lin (-). HSCs [Hematopoietic stem cells] in murine cultures show phenotypic markers as being CD34-, CD150+, and Flt3- for LTR [long-term reconstitution]. These phenotypic markers are used when purifying hematopoietic stem cells from the many other differentiated cells in the bone marrow.
Sca-1 stands for "Stem cells antigen-1". It consist of 18-kDa mouse glycosyl phosphatidylinositol-anchored cell surface protein (GPI-AP) of the LY6 gene family. It is the common biological marker used to identify hematopoietic stem cell (HSC) along with other markers.

Islet resident macrophages are the predominant myeloid cell of the pancreatic islets of langerhans.
OP9 cells are a cell line derived from mouse bone marrow stromal cells (mesenchyme). These cells are now characterized as stem cells. When co-cultured with embryonic stem cells (ESC), OP9 cells can induce ESC to differentiate into blood cells by serving as a feeder layer. They have the potential to be used in cell therapy, regenerative medicine and as immunomodulators.
References
- ↑ Civin CI. Strauss LC. Brovall C. Fackler MJ. Schwartz JF. Shaper JH. (1984). "Antigenic analysis of haematopoiesis:III. hematopoietic cell surface antigen defined by a monoclonal antibody raised against KG-1a cells". Journal of Immunology. 133 (1): 157–65. doi:10.4049/jimmunol.133.1.157.
- ↑ Tindle RW, Nichols RA, Chan L, Campana D, Catovsky D, Birnie GD (1985). "A novel monoclonal antibody BI-3C5 recognises myeloblasts and non-B non-T lymphoblasts in acute leukaemias and CGL blast crises, and reacts with immature cells in normal bone marrow". Leukemia Research. 9 (1): 1–9. doi:10.1016/0145-2126(85)90016-5. PMID 3857402.
- ↑ Tindle RW, Katz F, Martin H, Watt S, Catovsky D, Janossy G, Greaves M (1987). "BI-3C5 (CD34) defines multipotential and lineage restricted progenitor cells and their leukaemic counterparts". Leucocyte Typing III: White Cell Differentiation Antigens: 654–55.
- ↑ Loken MR, Shah VO, Civin CI (1987). "Characterization of myeloid antigens on human bone marrow using multicolour immunofluorescence". Leucocyte Typing III: White Cell Differentiation Antigens. pp. 630–35.
- ↑ "Stemcells Redirection". stemcells.nih.gov. Archived from the original on 2011-05-25. Retrieved 2019-07-08.
- ↑ Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE (September 1998). "A newly discovered class of human hematopoietic cells with SCID-repopulating activity". Nature Medicine. 4 (9): 1038–45. doi:10.1038/2023. PMID 9734397.
- ↑ Guo Y, Lübbert M, Engelhardt M (2003). "CD34- hematopoietic stem cells: current concepts and controversies". Stem Cells. 21 (1): 15–20. doi: 10.1634/stemcells.21-1-15 . PMID 12529547.
- ↑ Doi H, Inaba M, Yamamoto Y, Taketani S, Mori SI, Sugihara A, Ogata H, Toki J, Hisha H, Inaba K, Sogo S, Adachi M, Matsuda T, Good RA, Ikehara S (March 1997). "Pluripotent hemopoietic stem cells are c-kit<low". Proceedings of the National Academy of Sciences of the United States of America. 94 (6): 2513–7. Bibcode:1997PNAS...94.2513D. doi: 10.1073/pnas.94.6.2513 . PMC 20119 . PMID 9122226.
- ↑ Weksberg DC, Chambers SM, Boles NC, Goodell MA (February 2008). "CD150- side population cells represent a functionally distinct population of long-term hematopoietic stem cells". Blood. 111 (4): 2444–51. doi:10.1182/blood-2007-09-115006. PMC 2234069 . PMID 18055867.
- ↑ Van Zant G (2005). "Stem cell markers: less is more!". Blood. 107 (3): 855–56. doi: 10.1182/blood-2005-11-4400 .
- ↑ Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ (July 2005). "SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells". Cell. 121 (7): 1109–21. doi: 10.1016/j.cell.2005.05.026 . PMID 15989959.
- ↑ Muller-Sieburg, Christa E; Whitlock, Cheryl A; Weissman, Irving L (1986). "Isolation of two early B lymphocyte progenitors from mouse marrow: A committed Pre-Pre-B cell and a clonogenic Thy-1lo hematopoietic stem cell". Cell. 44 (4): 653–662. doi:10.1016/0092-8674(86)90274-6. PMID 2868799.
- ↑ Weissman, Irving L; Shizuru, Judith A (2008). "The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases". Blood. 112 (9): 3543–3553. doi:10.1182/blood-2008-08-078220. PMC 2574516 . PMID 18948588 – via ASH Publications.