
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles.

A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.

Acibenzolar-S-methyl is the ISO common name for an organic compound that is used as a fungicide. Unusually, it is not directly toxic to fungi but works by inducing systemic acquired resistance, the natural defence system of plants.

The Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids.

1,4-Dioxin (also referred as dioxin or p-dioxin) is a heterocyclic, organic, non-aromatic compound with the chemical formula C4H4O2. There is an isomeric form of 1,4-dioxin, 1,2-dioxin (or o-dioxin). 1,2-Dioxin is very unstable due to its peroxide-like characteristics.
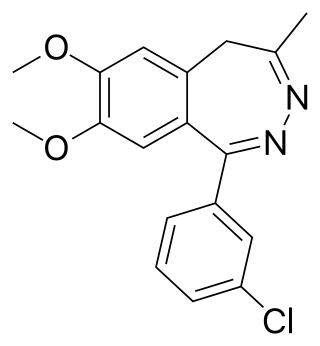
Girisopam is a drug which is a 2,3-benzodiazepine derivative, related to tofisopam and zometapine. It has selective anxiolytic action with no sedative, anticonvulsant or muscle relaxant effects.

Asphodeline is a genus of perennial plants in the family Asphodelaceae, first described as a genus in 1830. It is native to the eastern Mediterranean region and the Middle East from Italy and Algeria east to Iran.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.
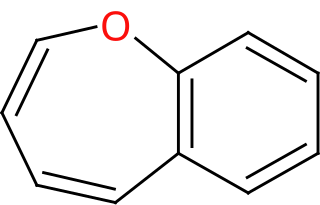
Benzoxepin is an oxygen-containing bicyclic molecule consisting of an oxepin ring and a benzene ring. There are three isomers, varying in where the oxygen is positioned in the oxepin heterocycle relative where the benzene is fused to it.

Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.

Ayhan Ulubelen was a Turkish analytical chemist. She was a member of the Turkish Academy of Sciences. Ulubelen contributed to the isolation and testing of natural products from Turkish plants relevant to spontaneous abortion, cancer, HIV, and diabetes.
Asphodeline tenuior, the thin asphodeline, is a species of plant in the family Asphodelaceae, subfamily Asphodeloideae. It is native to the Caucasus, as well as from eastern Turkey and northwestern Iran. Within Russia, it is known from eastern Krasnodar Krai, Karachay-Cherkessia, Stavropol Krai and western Kabardino-Balkaria. It can be found on stony slopes and scree on limestone and sandstone, from elevations of 500–1,000 m. It is threatened by habitat loss and degradation, due to lime pits, slope terracing and cattle pasturing.
- Asphodeline tenuior var. puberulentaTuzlaci - eastern Turkey
- Asphodeline tenuior subsp. tenuiflora(K.Koch) Tuzlaci - Turkey, Iran, south Caucasus
- Asphodeline tenuior subsp. tenuior - north and south Caucasus

3-Benzoxepin is an annulated ring system with an aromatic benzene ring and a non-aromatic, unsaturated, oxygen-containing seven-membered heterocyclic oxepin. The first synthesis was described by Karl Dimroth and coworkers in 1961. It is one of the three isomers of the benzoxepins.

Cyanogen azide, N3CN or CN4, is an azide compound of carbon and nitrogen which is an oily, colourless liquid at room temperature. It is a highly explosive chemical that is soluble in most organic solvents, and normally handled in dilute solution in this form. It was first synthesised by F. D. Marsh at DuPont in the early 1960s.

Cyanuric bromide is a heterocyclic compound with formula C3N3Br3. It contains a six-membered ring of alternating nitrogen and carbon atoms, with a bromine atom attached to each carbon. It is formed by the spontaneous trimerisation of cyanogen bromide.

Oxalyl dicyanide is a chemical compound with the formula C4N2O2.
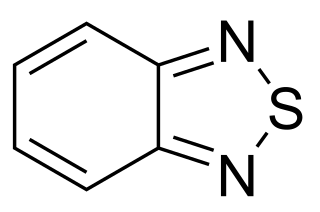
2,1,3-Benzothiadiazole is a bicyclic molecule composed of a benzene ring that is fused to a 1,2,5-thiadiazole.

1,2,3-Benzothiadiazole is a bicyclic aromatic chemical composed of a benzene ring that is fused to a 1,2,3-thiadiazole. A colorless solid, the compound is soluble in organic solvents.


















