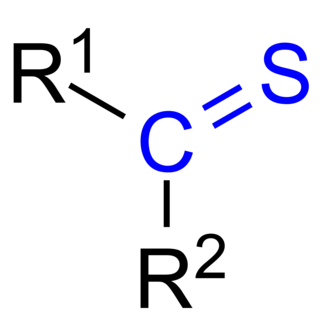A thiol or thiol derivative is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The –SH functional group itself is referred to as either a thiol group or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a portmanteau of "thio-" + "alcohol", with the first word deriving from Greek θεῖον (theion) meaning "sulfur".

An organic sulfide or thioether is a functional group in organosulfur chemistry with the connectivity C–S–C as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.

Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene (or their equivalents). An alkyl group is a piece of a molecule with the general formula CnH2n+1, where n is the integer depicting the number of carbons linked together. For example, a methyl group (n = 1, CH3) is a fragment of a methane molecule (CH4). Alkylating agents use selective alkylation by adding the desired aliphatic carbon chain to the previously chosen starting molecule. This is one of many known chemical syntheses. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character.
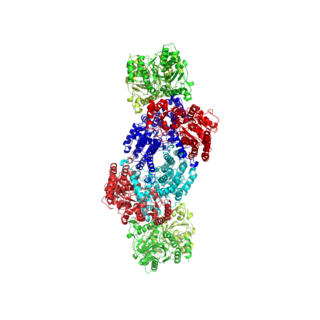
Nitrogenases are enzymes (EC 1.18.6.1EC 1.19.6.1) that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria). These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a key step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules (nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase.

Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: anions and organic polysulfides. Anions have the general formula S2−
n. These anions are the conjugate bases of the hydrogen polysulfides H2Sn. Organic polysulfides generally have the formulae RSnR, where R = alkyl or aryl.

A sulfoxide is a chemical compound containing a sulfinyl (SO) functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are the oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

Lithium sulfide is the inorganic compound with the formula Li2S. It crystallizes in the antifluorite motif, described as the salt (Li+)2S2−. It forms a solid yellow-white deliquescent powder. In air, it easily hydrolyses to release hydrogen sulfide (rotten egg odor).
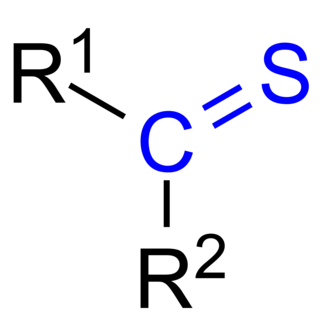
Thioketones (also known as thiones or thiocarbonyls) are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix "thio-" in the name of the functional group. Unhindered alkylthioketones typically tend to form polymers or rings.
The Corey–Kim oxidation is an oxidation reaction used to synthesise aldehydes and ketones from primary and secondary alcohols. It is named for American chemist and Nobel Laureate Elias James Corey and Korean-American chemist Choung Un Kim.
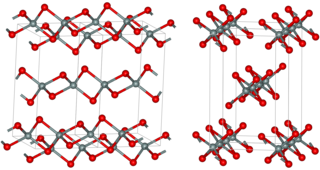
Silicon sulfide is the inorganic compound with the formula SiS2. Like silicon dioxide, this material is polymeric, but it adopts a 1-dimensional structure quite different from the usual forms of SiO2.
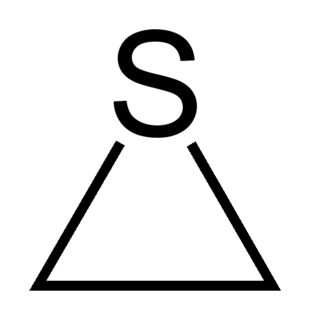
Thiirane, more commonly known as ethylene sulfide, is the cyclic chemical compound with the formula C2H4S. It is the smallest sulfur-containing heterocycle and the simplest episulfide. Like many organosulfur compounds, this species has a stench. Thiirane is also used to describe any derivative of the parent ethylene sulfide.

A dithiocarbamate is a functional group in organic chemistry. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms.

Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as pesticides and drugs. They are typically white or pale yellow solids that are soluble in organic solvents.
Organosilver chemistry in chemistry is the study of organometallic compounds containing a carbon to silver chemical bond and the study of silver as catalyst in organic reactions. In the group 11 elements silver is the element below copper. The chemistries have much in common but organosilver catalysis is much less common (mostly academic study) than organocopper chemistry due both to the relatively high price of silver and to the poor thermal stability of organosilver compounds. The oxidation state for silver in organosilver compounds in exclusively +1 with the notable exception of Ag(III) in the trifluoromethyl silver anion Ag(CF3)4− because of the electron-withdrawing effect of the trifluoromethyl groups. Poor thermal stability is reflected in decomposition temperatures of AgMe (-50 °C) versus CuMe (-15 °C) and PhAg (74 °C) vs PhCu (100 °C).
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.

Thiosulfurous acid is a low oxidation state (+1) sulfur acid. It is the equivalent acid for disulfur monoxide. Thiosulfurous acid is formed by reacting hydrogen sulfide and sulfur dioxide at room temperature. The thiosulfurous acid molecule is unstable when condensed, reacting with itself.

Dimethylthiocarbamoyl chloride is an organosulfur compound with the formula (CH3)2NC(S)Cl. A yellow solid, it is often encountered as a yellow syrup. It is a key reagent in the synthesis of arylthiols via the Newman-Kwart rearrangement.
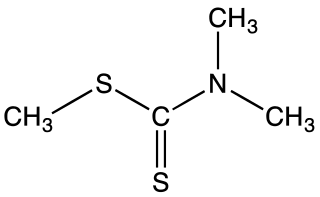
Methyl dimethyldithiocarbamate is the organosulfur compound with the formula (CH3)2NC(S)SCH3. It is the one of simplest dithiocarbamic esters. It is a white volatile solid that is poorly soluble in water but soluble in many organic solvents. It was once used as a pesticide.