Related Research Articles
Evidence-based medicine (EBM) is "the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients". The aim of EBM is to integrate the experience of the clinician, the values of the patient, and the best available scientific information to guide decision-making about clinical management. The term was originally used to describe an approach to teaching the practice of medicine and improving decisions by individual physicians about individual patients.

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments.

Cochrane is a British international charitable organisation formed to organise medical research findings to facilitate evidence-based choices about health interventions involving health professionals, patients and policy makers. It includes 53 review groups that are based at research institutions worldwide. Cochrane has approximately 30,000 volunteer experts from around the world.
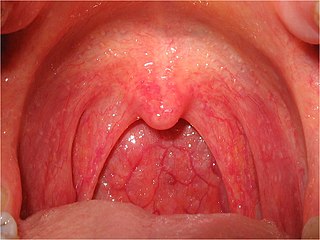
Sore throat, also known as throat pain, is pain or irritation of the throat. Usually, causes of sore throat include

A medical guideline is a document with the aim of guiding decisions and criteria regarding diagnosis, management, and treatment in specific areas of healthcare. Such documents have been in use for thousands of years during the entire history of medicine. However, in contrast to previous approaches, which were often based on tradition or authority, modern medical guidelines are based on an examination of current evidence within the paradigm of evidence-based medicine. They usually include summarized consensus statements on best practice in healthcare. A healthcare provider is obliged to know the medical guidelines of his or her profession, and has to decide whether to follow the recommendations of a guideline for an individual treatment.

A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic. A systematic review extracts and interprets data from published studies on the topic, then analyzes, describes, and summarizes interpretations into a refined conclusion. For example, a systematic review of randomized controlled trials is a way of summarizing and implementing evidence-based medicine.

Gordon Henry Guyatt is a Canadian physician who is Distinguished University Professor in the Departments of Health Research Methods, Evidence and Impact and Medicine at McMaster University in Hamilton, Ontario. He is known for his leadership in evidence-based medicine, a term that first appeared in a single-author paper he published in 1991. Subsequently, a 1992 JAMA article that Guyatt led proved instrumental in bringing the concept of evidence-based medicine to the world's attention.[2] In 2007, The BMJ launched an international election for the most important contributions to healthcare. Evidence-based medicine came 7th, ahead of the computer and medical imaging. [3][4] Guyatt's concerns with the role of the medical system, social justice, and medical reform remain central issues that he promoted in tandem with his medical work. On October 9, 2015, he was named to the Canadian Medical Hall of Fame.
A hierarchy of evidence is a heuristic used to rank the relative strength of results obtained from scientific research. There is broad agreement on the relative strength of large-scale, epidemiological studies. More than 80 different hierarchies have been proposed for assessing medical evidence. The design of the study and the endpoints measured affect the strength of the evidence. In clinical research, the best evidence for treatment efficacy is mainly from meta-analyses of randomized controlled trials (RCTs). Systematic reviews of completed, high-quality randomized controlled trials – such as those published by the Cochrane Collaboration rank the same as systematic review of completed high-quality observational studies in regard to the study of side effects. Evidence hierarchies are often applied in evidence-based practices and are integral to evidence-based medicine (EBM).
In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient, but usually describe an unusual or novel occurrence. Some case reports also contain a literature review of other reported cases. Case reports are professional narratives that provide feedback on clinical practice guidelines and offer a framework for early signals of effectiveness, adverse events, and cost. They can be shared for medical, scientific, or educational purposes.
David Lawrence Sackett was an American-Canadian physician and a pioneer in evidence-based medicine. He is known as one of the fathers of Evidence-Based Medicine. He founded the first department of clinical epidemiology in Canada at McMaster University, and the Oxford Centre for Evidence-Based Medicine. He is well known for his textbooks Clinical Epidemiology and Evidence-Based Medicine.

The Users’ Guides to the Medical Literature is a series of articles originally published in the Journal of the American Medical Association, later rewritten and compiled in a textbook, now in its third edition. The guides provide practical, clinician-friendly advice on all aspects of evidence-based medicine.
Critical appraisal in evidence based medicine, is the use of explicit, transparent methods to assess the data in published research, applying the rules of evidence to factors such as internal validity, adherence to reporting standards, conclusions, generalizability and risk-of-bias. Critical appraisal methods form a central part of the systematic review process. They are used in evidence synthesis to assist clinical decision-making, and are increasingly used in evidence-based social care and education provision.
The Bradford Hill criteria, otherwise known as Hill's criteria for causation, are a group of nine principles that can be useful in establishing epidemiologic evidence of a causal relationship between a presumed cause and an observed effect and have been widely used in public health research. They were established in 1965 by the English epidemiologist Sir Austin Bradford Hill.
Alessandro Liberati was an Italian healthcare researcher and clinical epidemiologist, and founder of the Italian Cochrane Centre.
The minimal important difference (MID) or minimal clinically important difference (MCID) is the smallest change in a treatment outcome that an individual patient would identify as important and which would indicate a change in the patient's management.
There is a history of clinical research done on glycosaminoglycans, especially glucosamine and chondroitin, for the treatment of arthritis. Since glucosamine is a precursor for glycosaminoglycans, and glycosaminoglycans are major components of cartilage, ingesting glucosamine might nourish joints, and thereby alleviate arthritis symptoms.
Holger Jens Schünemann is a physician and professor of medicine and Clinical epidemiology. From 1 February 2009 to 30 June 2019 he was the chair of the Department of Health Research Methods, Evidence, and Impact at McMaster University in Hamilton, Canada, where he now works as full professor.
Journalology is the scholarly study of all aspects of the academic publishing process. The field seeks to improve the quality of scholarly research by implementing evidence-based practices in academic publishing. The term "journalology" was coined by Stephen Lock, the former editor-in-chief of the BMJ. The first Peer Review Congress, held in 1989 in Chicago, Illinois, is considered a pivotal moment in the founding of journalology as a distinct field. The field of journalology has been influential in pushing for study pre-registration in science, particularly in clinical trials. Clinical trial registration is now expected in most countries. Journalology researchers also work to reform the peer review process.
A fact box is a simplified display format that presents evidence based data about the benefits and harms of medical treatments, screenings or interventions.
References
- ↑ Schünemann, HJ; Best, D; Vist, G; Oxman, AD (2003). "Letters, numbers, symbols, and words: How best to communicate grades of evidence and recommendations?". Canadian Medical Association Journal. 169 (7): 677–80.
- ↑ Guyatt, GH; Oxman, AD; Vist, GE; Kunz, R; Falck-Ytter, Y; Alonso-Coello, P; Schünemann, HJ (2008). "GRADE: an emerging consensus on rating quality of evidence and strength of recommendation". BMJ. 336 (7650): 924–26. doi:10.1136/bmj.39489.470347.ad. PMC 2335261 . PMID 18436948.
- ↑ Guyatt, GH; Oxman, AD; Schünemann, HJ; Tugwell, P; Knotterus, A (2011). "GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology". Journal of Clinical Epidemiology. 64 (4): 380–382. doi:10.1016/j.jclinepi.2010.09.011. PMID 21185693.
- ↑ "GRADE home". Gradeworkinggroup.org. Retrieved 16 August 2019.
- ↑ Andrews, J; Guyatt, GH; Oxman, AD; Alderson, P; Dahm, P; Falck-Ytter, Y; Nasser, M; Meerpohl, J; Post, PN; Kunz, R; Brozek, J; Vist, G; Rind, D; Akl, EA; Schünemann, HJ (2013). "GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations". Journal of Clinical Epidemiology. 66 (7): 719–725. doi:10.1016/j.jclinepi.2012.03.013. PMID 23312392.
- ↑ Balshem, H; Helfand, M; Schünemann, HJ; Oxman, AD; Kunz, R; Brozek, J; Vist, GE; Falck-Ytter, Y; Meerpohl, J; Norris, S; Guyatt, GH (April 2011). "GRADE guidelines 3: rating the quality of evidence - introduction". Journal of Clinical Epidemiology. 64 (4): 401–406. doi:10.1016/j.jclinepi.2010.07.015. PMID 21208779.
- ↑ Reed Siemieniuk and Gordon Guyatt. "What is GRADE?". BMJ Best Practice. Retrieved 2020-07-02.
- ↑ "GRADEpro". Gradepro.org. Retrieved 16 August 2019.
- ↑ Schünemann, HJ; Oxman, AD; Brozek, J; Glasziou, P; Jaeschke, R; Vist, G; Williams, J; Kunz, R; Craig, J; Montori, V; Bossuyt, P; Guyatt, GH (2008). "GRADEing the quality of evidence and strength of recommendations for diagnostic tests and strategies". BMJ. 336 (7653): 1106–1110. doi:10.1136/bmj.39500.677199.ae. PMC 2386626 . PMID 18483053.
- ↑ Brozek, JL; Akl, EA; Jaeschke, R; Lang, DM; Bossuyt, P; Glasziou, P; Helfand, M; Ueffing, E; Alonso-Coello, P; Meerpohl, J; Phillips, B; Horvath, AR; Bousquet, J; Guyatt, GH; Schünemann, HJ (2009). "Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies". Allergy. 64 (8): 1109–16. doi:10.1111/j.1398-9995.2009.02083.x. PMID 19489757. S2CID 8865010.
- ↑ Iorio, A; Spencer, FA; Falavigna, M; Alba, C; Lang, E; Burnand, B; McGinn, T; Hayden, J; Williams, K; Shea, B; Wolff, R; Kujpers, T; Perel, P; Vandvik, PO; Glasziou, P; Schünemann, H; Guyatt, G (2015). "Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients". BMJ. 350: h870. doi: 10.1136/bmj.h870 . PMID 25775931.
- ↑ Spencer, FA; Iorio, A; You, J; Murad, MH; Schünemann, HJ; Vandvik, PO; Crowther, MA; Pottie, K; Lang, ES; Meerpohl, JJ; Falck-Ytter, Y; Alonso-Coello, P; Guyatt, GH (2012). "Uncertainties in baseline risk estimates and confidence in treatment effects". BMJ. 14: 345. doi: 10.1136/bmj.e7401 . PMID 23152569.
- ↑ Puhan, MA; Schünemann, HJ; Murad, MH; Li, T; Brignardello-Petersen, R; Singh, JA; Kessels, AG; Guyatt, GH (2014). "A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis". BMJ. 24: 349. doi: 10.1136/bmj.g5630 . PMID 25252733.
- ↑ Burford, BJ; Rehfuess, E; Schünemann, HJ; Akl, EA; Waters, E; Armstrong, R; Thomson, H; Doyle, J; Pettman, T (2012). "Assessing evidence in public health: the added value of GRADE". J Public Health. 34 (4): 631–5. doi: 10.1093/pubmed/fds092 . PMID 23175858.
- ↑ "GRADEpro". Gradepro.org. Retrieved 16 August 2019.
- ↑ "The Saudi Center for Evidence Based Healthcare (EBHC) - Clinical Practice Guidelines". 2016-02-25. Archived from the original on 2016-02-25. Retrieved 2021-02-19.