
Pharmacology is a science of medical drug and medication, including a substance's origin, composition, pharmacokinetics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.

David HealyFRCPsych, a professor of psychiatry at Bangor University in the United Kingdom, is a psychiatrist, psychopharmacologist, scientist and author. His main areas of research are the contribution of antidepressants to suicide, conflict of interest between pharmaceutical companies and academic medicine, and the history of pharmacology. Healy has written more than 150 peer-reviewed articles, 200 other articles, and 20 books, including The Antidepressant Era, The Creation of Psychopharmacology, The Psychopharmacologists Volumes 1–3, Let Them Eat Prozac and Mania: A Short History of Bipolar Disorder.
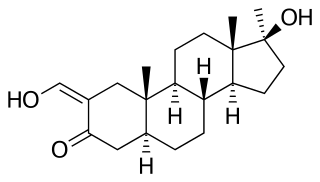
Oxymetholone, sold under the brand names Anadrol and Anapolon among others, is an androgen and anabolic steroid (AAS) medication which is used primarily in the treatment of anemia. It is also used to treat osteoporosis, HIV/AIDS wasting syndrome, and to promote weight gain and muscle growth in certain situations. It is taken by mouth.

Sodium oxybate, sold under the brand name Xyrem among others, is a medication used to treat symptoms of narcolepsy: sudden muscle weakness and excessive daytime sleepiness. It is used sometimes in France and Italy as an anesthetic given intravenously; it is also approved and used in Italy and in Austria to treat alcohol dependence and alcohol withdrawal syndrome.
Pharmacy and Therapeutics (P&T) is a committee at a hospital or a health insurance plan that decides which drugs will appear on that entity's drug formulary. The committee usually consists of healthcare providers involved in prescribing, dispensing, and administering medications, as well as administrators who evaluate medication use. They must weigh the costs and benefits of each drug and decide which ones provide the most efficacy per dollar. This is one aspect of pharmaceutical policy. P&T committees utilize an evidence-based approach to drive change within health systems/plans by changing existing policies and bringing up-to-date research to support medical decision-making.

Mecamylamine is a non-selective, non-competitive antagonist of the nicotinic acetylcholine receptors (nAChRs) that was introduced in the 1950s as an antihypertensive drug. In the United States, it was voluntarily withdrawn from the market in 2009 but was brought to market in 2013 as Vecamyl and eventually was marketed by Turing Pharmaceuticals.

Clindamycin/benzoyl peroxide is a topical gel used for the treatment of acne. It is a fixed-dose combination of clindamycin, as the phosphate, an antibiotic, and benzoyl peroxide, an antiseptic.

The Center for Drug Evaluation and Research is a division of the U.S. Food and Drug Administration (FDA) that monitors most drugs as defined in the Food, Drug, and Cosmetic Act. Some biological products are also legally considered drugs, but they are covered by the Center for Biologics Evaluation and Research. The center reviews applications for brand name, generic, and over the counter pharmaceuticals, manages US current Good Manufacturing Practice (cGMP) regulations for pharmaceutical manufacturing, determines which medications require a medical prescription, monitors advertising of approved medications, and collects and analyzes safety data about pharmaceuticals that are already on the market.

The Compendium of Pharmaceuticals and Specialties: The Canadian Drug Reference for Health Professionals, more commonly known by its abbreviation CPS, is a reference book that contains drug monographs and numerous features which help healthcare professionals prescribe and use drugs safely and appropriately. A print version of CPS is released annually by the Canadian Pharmacists Association (CPhA). The CPhA is a nonprofit organization that advocates for pharmacists in Canada. An external Editorial Advisory Committee of Canadian physicians and pharmacists advises CPhA about the strategic direction of their publications including CPS. CPS is also available online by subscription at www.pharmacists.ca. Most of the drug monographs in CPS are provided by manufacturers, though numerous monographs—usually for drugs which are available as generic brands— are written by CPhA editorial staff and peer reviewed. 2010 marked the 50th anniversary of the first edition of CPS.
Expert Opinion on Investigational Drugs is a monthly peer-reviewed medical journal covering developments in pharmaceutical research, from animal studies through to early clinical investigation. The journal's scope includes therapeutics in many areas: pulmonary-allergy, dermatology, gastrointestinal, arthritis, infectious disorders, endocrine and metabolic, central and peripheral nervous system, cardiovascular and renal, and oncology.
Expert Opinion on Therapeutic Targets is a monthly peer-reviewed medical journal publishing review articles and original papers on recently identified novel molecular drug targets across all therapy areas. It was originally established as Emerging Therapeutic Targets in 1997, changing to its current name in 2001.

Drugs is a peer-reviewed medical journal published by Adis International that covers topics in drugs and therapeutics. Besides research articles, the journal also publishes "Adis Drug Evaluations and AdisInsight Reports", evidence-based, single-agent reviews.
Critical Path Institute (C-Path) is a non-profit organization created to improve the drug development process; its consortia include more than 1,600 scientists from government regulatory and research agencies, academia, patient organizations, and bio-pharmaceutical companies.

United Therapeutics Corporation is an American publicly traded biotechnology company and public benefit corporation listed on the NASDAQ under the symbol UTHR. It develops novel, life-extending technologies for patients in the areas of lung disease and organ manufacturing. United Therapeutics is co-headquartered in Silver Spring, Maryland and Research Triangle Park, North Carolina, with additional facilities in Magog and Bromont, Quebec; Melbourne and Jacksonville, Florida; Blacksburg, Virginia; and Manchester, New Hampshire.
Prescrire also known as La Revue Prescrire is a monthly medical journal in French which addresses developments in diseases, medications, and in medical techniques and technologies. Prescrire contains no advertising, and is internally financed by subscriptions and the sale of various personnel training. In contrast to many traditional medical journals, which review and publish articles submitted by external researchers, Prescrire mainly publishes reviews prepared by its own staff.
The Institute for Clinical and Economic Review (ICER) is a Boston-based independent nonprofit organization that seeks to place a value on medical care by providing comprehensive clinical and cost-effectiveness analyses of treatments, tests, and procedures.
Steven M. Paul is an American neuroscientist and pharmaceutical executive. As of 2021, Paul serves as the CEO, president and chairman of Karuna Therapeutics.

Zuranolone, sold under the brand name Zurzuvae, is a medication used for the treatment of postpartum depression. It is taken by mouth.










