Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes.
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. A polyketide, polyether derivative of a C38 fatty acid, okadaic acid and other members of its family have shined light upon many biological processes both with respect to dinoflagellete polyketide synthesis as well as the role of protein phosphatases in cell growth.

Isatin, also known as tribulin, is an organic compound derived from indole with formula C8H5NO2. The compound was first obtained by Otto Linné Erdman and Auguste Laurent in 1840 as a product from the oxidation of indigo dye by nitric acid and chromic acids.

Terthiophene is the organic compound with the formula [C4H3S]2C4H2S. It is an oligomer of the heterocycle thiophene, a shorter oligomer is dithienyl, and the parent polymer is polythiophene. In the most common isomer of terthiophene, two thienyl groups are connected via their 2 positions to a central thiophene, also at the carbon atoms flanking the sulfur.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.

Tropolone is an organic compound with the chemical formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.

Pulvinone, an organic compound belonging to the esters, lactones, alcohols and butenolides classes, is a yellow crystalline solid. Although the pulvinone is not a natural product, several naturally occurring hydroxylated derivatives are known. These hydroxylated pulvinones are produced by fungal species, such as the in Europe common Larch Bolete, or by moulds such as Aspergillus terreus.

Heinz Falk is professor emeritus for organic chemistry at Johannes Kepler University of Linz and editor of "Progress in the Chemistry of Organic Natural Compounds". His research is focused on structural analysis, synthesis, stereochemistry and photochemistry of plant and animal photosensitizing and photosensory pigments, such as hypericin.

Hydnellum is a genus of tooth fungi in the family Bankeraceae. Widely distributed in the Northern Hemisphere, the genus contains around 40 species. The fruitbodies of its members grow by slowly enveloping nearby bits of grass and vegetation. There is great variability in the form of Hydnellum fruitbodies, which are greatly influenced by environmental conditions such as rainfall and humidity, drying winds, and temperature. They are too tough and woody to eat comfortably. Several species have become the focus of increasing conservation concern following widespread declines in abundance.

Hydnellum aurantiacum is an inedible fungus, commonly known as the orange spine or orange Hydnellum for its reddish orange or rusty red colored fruit bodies. Like other tooth fungi, it bears a layer of spines rather than gills on the underside of the cap. Due to substantial declines in sightings, this species is listed as critically endangered in the United Kingdom.

Polyozellus is a fungal genus in the family Thelephoraceae, a grouping of mushrooms known collectively as the leathery earthfans. A monotypic genus, it contains the single species Polyozellus multiplex, first described in 1899, and commonly known as the blue chanterelle, the clustered blue chanterelle, or, in Alaska, the black chanterelle. The distinctive fruit body of this species comprises blue- to purple-colored clusters of vase- or spoon-shaped caps with veiny wrinkles on the undersurface that run down the length of the stem.

Thelephora is a genus of fungi in the family Thelephoraceae. The genus has a widespread distribution and contains about 50 species. Fruit bodies of species are leathery, usually brownish at maturity, and range in shape from coral-like tufts to having distinct caps. Almost all species in the genus are thought to be inedible, but Thelephora ganbajun is a gourmet fungus in Yunnan province of southwest China.

Haematopodin is the more stable breakdown product of Haematopodin B. Both compounds are found in the mushroom Mycena haematopus, although haematopodin only occurs in trace amounts in fresh fruit bodies. Similar pigments, known as batzellins and damirones, have been found in sea sponges. A chemical synthesis for haematopodin was reported in 1996. Key steps in the synthesis involved the addition of 3-[(2,4-dimethoxybenzyl)amino]-1-propanol to the indolo-6,7-quinone and cyclization of the resulting adduct with trifluoroacetic acid.
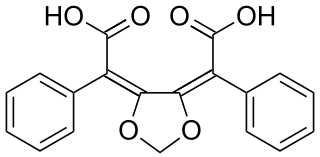
Ustalic acid is a naturally occurring chemical compound found in the poisonous mushroom Tricholoma ustale.
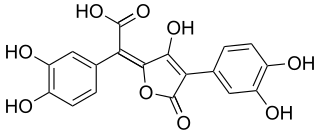
Variegatic acid is an orange pigment found in some mushrooms. It is responsible for the bluing reaction seen in many bolete mushrooms when they are injured. When mushroom tissue containing variegatic acid is exposed to air, the chemical is enzymatically oxidized to blue quinone methide anions, specifically chinonmethid anions. It is derived from xerocomic acid, which is preceded by atromentic acid and atromentin, and its genetic basis is unknown. In its oxidized form is variegatorubin, similar to xerocomorubin.

Norbadione A is a pigment found in the bay bolete mushroom. A polyphenol, norbadione A is related to a family of mushroom pigments known as pulvinic acids. The molecule has also been reported as a potassium salt from the mushrooms Pisolithus tinctorius and Chalciporus piperatus.

Xerocomorubin is a pigment from the fungus order Boletales. It is the oxidized form of isoxerocomic acid. Air oxidation is responsible its formation, and it oxidizes faster to a similar pulvinic acid type pigment oxidized variant, variegatorubin. The long wavelength has an absorption at 497 nm, 106 nm higher than its precursor isoxerocomic acid. Synthesis experiments have shown tetra-acetylation by acetic anhydride and sulfuric acid. Although xerocomorubin and variegatorubin give off the same deep red color and could simultaneously occur in a mushroom, extracts from the deep red colored mushroom Boletus rubellus Krombh. identified only variegatorubin by thin layer chromatography (TLC), leading to the question the natural abundance of xerocomorubin.

Thelephora terrestris is an inedible species of fungus in the Basidiomycota phylum. It is commonly known by the name Common Fiber Vase because of its circular and overlapping cap. As well, it has also been called the Earthfan fungus.


















