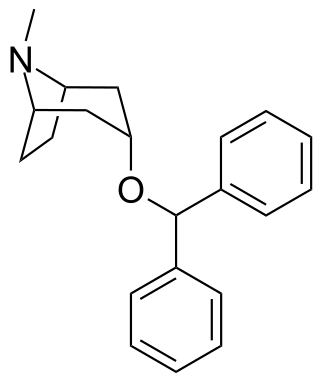
Benzatropine (INN), known as benztropine in the United States and Japan, is a medication used to treat movement disorders like parkinsonism and dystonia, as well as extrapyramidal side effects of antipsychotics, including akathisia. It is not useful for tardive dyskinesia. It is taken by mouth or by injection into a vein or muscle. Benefits are seen within two hours and last for up to ten hours.

Atomoxetine, formerly sold under the brand name Strattera, is a selective norepinephrine reuptake inhibitor (sNRI) medication used to treat attention deficit hyperactivity disorder (ADHD) and, to a lesser extent, cognitive disengagement syndrome (CDS). It may be used alone or along with stimulant medication. It enhances the executive functions of self-motivation, sustained attention, inhibition, working memory, reaction time, and emotional self-regulation. Use of atomoxetine is only recommended for those who are at least six years old. It is taken orally. The effectiveness of atomoxetine is comparable to the commonly prescribed stimulant medication methylphenidate.
Psychedelic therapy refers to the proposed use of psychedelic drugs, such as psilocybin, ayahuasca, LSD, psilocin, mescaline (peyote), DMT, 5-MeO-DMT, Ibogaine, MDMA, to treat mental disorders. As of 2021, psychedelic drugs are controlled substances in most countries and psychedelic therapy is not legally available outside clinical trials, with some exceptions.
Progabide is an analogue and prodrug of γ-aminobutyric acid (GABA) used in the treatment of epilepsy. Via conversion into GABA, progabide behaves as an agonist of the GABAA, GABAB, and GABAA-ρ receptors.

Moclobemide, sold under the brand names Amira, Aurorix, Clobemix, Depnil and Manerix among others, is a reversible inhibitor of monoamine oxidase A (RIMA) drug primarily used to treat depression and social anxiety. It is not approved for use in the United States, but is approved in other Western countries such as Canada, the UK and Australia. It is produced by affiliates of the Hoffmann–La Roche pharmaceutical company. Initially, Aurorix was also marketed by Roche in South Africa, but was withdrawn after its patent rights expired and Cipla Medpro's Depnil and Pharma Dynamic's Clorix became available at half the cost.
Afterglow, when used in the context of recreational drug use, refers to positive physical and mental effects that linger after the main effects of a drug have subsided, or after the peak experience has subsided. This state is often characterized by feelings of detachment or increased psychological clarity. The term is most commonly associated with hallucinogens, particularly psychedelics and entactogens. Psychiatrist Walter Pahnke described afterglow as an “elevated and energetic mood with a relative freedom from concerns of the past and from guilt and anxiety.”

Sulpiride, sold under the brand name Dogmatil among others, is an atypical antipsychotic medication of the benzamide class which is used mainly in the treatment of psychosis associated with schizophrenia and major depressive disorder, and is sometimes used in low dosage to treat anxiety and mild depression.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

The Journal of Sex & Marital Therapy is a peer-reviewed scientific journal published by Routledge and formerly by Brunner/Mazel. Its editor-in-chief is R. Taylor Segraves.

Melperone is an atypical antipsychotic of the butyrophenone chemical class, making it structurally related to the typical antipsychotic haloperidol. It first entered clinical use in 1960s.

Diclofensine (Ro 8-4650) was developed by Hoffmann-La Roche in the 1970s in the search for a new antidepressant. It was found that the (S)-isomer was responsible for activity. Diclofensine is a stimulant drug which acts as a triple monoamine reuptake inhibitor, primarily inhibiting the reuptake of dopamine and norepinephrine, with affinities (Ki) of 16.8 nM, 15.7 nM, and 51 nM for DAT, NET, and SERT (dopamine, norepinephrine and serotonin transporters), respectively. It was found to be an effective antidepressant in human trials, with relatively few side effects, but was ultimately dropped from clinical development, possibly due to concerns about its abuse potential.
α-Methylserotonin (αMS), also known as α-methyl-5-hydroxytryptamine (α-methyl-5-HT) or 5-hydroxy-α-methyltryptamine (5-HO-αMT), is a tryptamine derivative closely related to the neurotransmitter serotonin (5-HT). It acts as a non-selective serotonin receptor agonist and has been used extensively in scientific research to study the function of the serotonin system.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.

BMY-14802, also known as BMS-181100, is a drug with antipsychotic effects which acts as both a sigma receptor antagonist and a 5-HT1A receptor agonist. It also has affinity for the 5-HT2 and D4 receptors. The drug reached phase III clinical trials for the treatment of psychosis but was never marketed.

Bay R 1531 is a tricyclic tryptamine derivative which acts as a selective serotonin receptor 5-HT1A agonist. It was researched unsuccessfully for the treatment of stroke but remains in use for scientific research.

Flubromazepam is a benzodiazepine derivative which was first synthesized in 1960, but was never marketed and did not receive any further attention or study until late 2012 when it appeared on the grey market as a novel designer drug.
Psychedelic microdosing involves consuming sub-threshold doses (microdoses) of serotonergic psychedelic drugs like LSD and psilocybin to potentially enhance creativity, energy, emotional balance, problem-solving abilities, and to address anxiety, depression, and addiction. This practice has gained popularity in the 21st century. A June 2024 report by the RAND Corporation suggests that among adults in the United States reporting the use of psilocybin in the past year, nearly half reported microdosing the last time they used it.

David Taylor FFRPS FRPharmS is a British professor. He is the head of the Pharmaceutical Sciences Clinical Academic Group within King's Health Partners. Taylor has been lead author and editor of the Maudsley Prescribing Guidelines in Psychiatry since 1994. In 2014, Taylor was named as one of the top 100 clinical leaders in the UK National Health Service.
In medicine, tapering is the practice of gradually reducing the dosage of a medication to reduce or discontinue it. Generally, tapering is done is to avoid or minimize withdrawal symptoms that arise from neurobiological adaptation to the drug.














