Related Research Articles
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

Size-exclusion chromatography, also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are commonly composed of dextran, agarose, or polyacrylamide polymers. The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc, which have been dissolved into liquid solutions.
Gel permeation chromatography (GPC) is a type of size-exclusion chromatography (SEC), that separates high molecular weight or colloidal analytes on the basis of size or diameter, typically in organic solvents. The technique is often used for the analysis of polymers. As a technique, SEC was first developed in 1955 by Lathe and Ruthven. The term gel permeation chromatography can be traced back to J.C. Moore of the Dow Chemical Company who investigated the technique in 1964. The proprietary column technology was licensed to Waters Corporation, who subsequently commercialized this technology in 1964. GPC systems and consumables are now also available from a number of manufacturers. It is often necessary to separate polymers, both to analyze them as well as to purify the desired product.
Micellar electrokinetic chromatography (MEKC) is a chromatography technique used in analytical chemistry. It is a modification of capillary electrophoresis (CE), extending its functionality to neutral analytes, where the samples are separated by differential partitioning between micelles and a surrounding aqueous buffer solution.

Column chromatography in chemistry is a chromatography method used to isolate a single chemical compound from a mixture. Chromatography is able to separate substances based on differential adsorption of compounds to the adsorbent; compounds move through the column at different rates, allowing them to be separated into fractions. The technique is widely applicable, as many different adsorbents can be used with a wide range of solvents. The technique can be used on scales from micrograms up to kilograms. The main advantage of column chromatography is the relatively low cost and disposability of the stationary phase used in the process. The latter prevents cross-contamination and stationary phase degradation due to recycling. Column chromatography can be done using gravity to move the solvent, or using compressed gas to push the solvent through the column.

Ion chromatography is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including small inorganic anions, large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one pH unit away from the isoelectric point of a protein.

Solid-phase extraction (SPE) is a solid-liquid extractive technique, by which compounds that are dissolved or suspended in a liquid mixture are separated, isolated or purified, from other compounds in this mixture, according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.
Chiral column chromatography is a variant of column chromatography that is employed for the separation of chiral compounds, i.e. enantiomers, in mixtures such as racemates or related compounds. The chiral stationary phase (CSP) is made of a support, usually silica based, on which a chiral reagent or a macromolecule with numerous chiral centers is bonded or immobilized.

Fast protein liquid chromatography (FPLC) is a form of liquid chromatography that is often used to analyze or purify mixtures of proteins. As in other forms of chromatography, separation is possible because the different components of a mixture have different affinities for two materials, a moving fluid (the mobile phase) and a porous solid (the stationary phase). In FPLC the mobile phase is an aqueous buffer solution. The buffer flow rate is controlled by a positive-displacement pump and is normally kept constant, while the composition of the buffer can be varied by drawing fluids in different proportions from two or more external reservoirs. The stationary phase is a resin composed of beads, usually of cross-linked agarose, packed into a cylindrical glass or plastic column. FPLC resins are available in a wide range of bead sizes and surface ligands depending on the application.
Reversed-phase Liquid chromatography (RP-LC) is a mode of liquid chromatography in which non-polar stationary phase and polar mobile phases are used for the separation of organic compounds. The vast majority of separations and analyses using High Performance Liquid Chromatography-HPLC in recent years are done using the Reversed Phase mode. In the Reversed Phase mode, the sample components are retained in the system, the more hydrophobic they are.
Mixed-mode chromatography (MMC), or multimodal chromatography, refers to chromatographic methods that utilize more than one form of interaction between the stationary phase and analytes in order to achieve their separation. What is distinct from conventional single-mode chromatography is that the secondary interactions in MMC cannot be too weak, and thus they also contribute to the retention of the solutes.
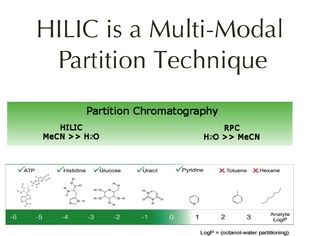
Hydrophilic interaction chromatography is a variant of normal phase liquid chromatography that partly overlaps with other chromatographic applications such as ion chromatography and reversed phase liquid chromatography. HILIC uses hydrophilic stationary phases with reversed-phase type eluents. The name was suggested by Andrew Alpert in his 1990 paper on the subject. He described the chromatographic mechanism for it as liquid-liquid partition chromatography where analytes elute in order of increasing polarity, a conclusion supported by a review and re-evaluation of published data.
Supercritical fluid chromatography (SFC) is a form of normal phase chromatography that uses a supercritical fluid such as carbon dioxide as the mobile phase. It is used for the analysis and purification of low to moderate molecular weight, thermally labile molecules and can also be used for the separation of chiral compounds. Principles are similar to those of high performance liquid chromatography (HPLC), however SFC typically utilizes carbon dioxide as the mobile phase; therefore the entire chromatographic flow path must be pressurized. Because the supercritical phase represents a state whereby bulk liquid and gas properties converge, supercritical fluid chromatography is sometimes called convergence chromatography. The idea of liquid and gas properties convergence was first envisioned by Giddings.
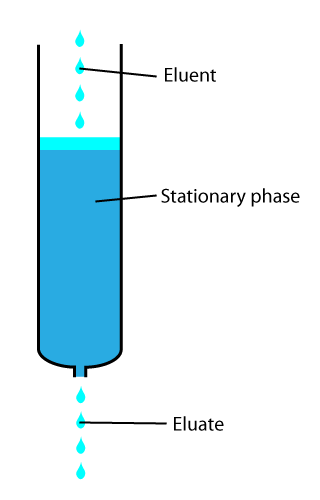
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
Displacement chromatography is a chromatography technique in which a sample is placed onto the head of the column and is then displaced by a solute that is more strongly sorbed than the components of the original mixture. The result is that the components are resolved into consecutive “rectangular” zones of highly concentrated pure substances rather than solvent-separated “peaks”. It is primarily a preparative technique; higher product concentration, higher purity, and increased throughput may be obtained compared to other modes of chromatography.
Poly(N-isopropylacrylamide) (variously abbreviated PNIPA, PNIPAM, PNIPAAm, NIPA, PNIPAA or PNIPAm) is a temperature-responsive polymer that was first synthesized in the 1950s. It can be synthesized from N-isopropylacrylamide which is commercially available. It is synthesized via free-radical polymerization and is readily functionalized making it useful in a variety of applications.

Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit drastic and discontinuous changes in their physical properties with temperature. The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, either an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST) exists.
A monolithic HPLC column, or monolithic column, is a column used in high-performance liquid chromatography (HPLC). The internal structure of the monolithic column is created in such a way that many channels form inside the column. The material inside the column which separates the channels can be porous and functionalized. In contrast, most HPLC configurations use particulate packed columns; in these configurations, tiny beads of an inert substance, typically a modified silica, are used inside the column. Monolithic columns can be broken down into two categories, silica-based and polymer-based monoliths. Silica-based monoliths are known for their efficiency in separating smaller molecules while, polymer-based are known for separating large protein molecules.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.
References
- ↑ Irene Tan, Farnoosh Roohi, Maria-Magdalena Titirici, Thermoresponsive polymers in liquid chromatography, Analytical Methods, 2012, Volume 4, pages 34-43.
- ↑ Hideko Kanazawa (2007). "Thermally responsive chromatographic materials using functional polymers". J. Sep. Sci. 30 (11): 1646–1656. doi:10.1002/jssc.200700093. PMID 17623446.
- ↑ Eri Ayano; Hideko Kanazawa (2006). "Aqueous chromatography system using temperature-responsive polymer-modified stationary phases". J. Sep. Sci. 29 (6): 738–749. doi:10.1002/jssc.200500485. PMID 16830486.
- ↑ Gewehr, Markus; Nakamura, Katsunori; Ise, Norio; Kitano, Hiromi (1992). "Gel permeation chromatography using porous glass beads modified with temperature-responsive polymers". Die Makromolekulare Chemie. 193 (1): 249–256. doi:10.1002/macp.1992.021930123. ISSN 0025-116X.
- ↑ Hideko Kanazawa; Yuki Kashiwase; Kazuo Yamamoto; Yoshikazu Matsushima; Akihiko Kikuchi; Yasuhisa Sakurai; Teruo Okano (1997). "Temperature-responsive liquid chromatography. 2. Effects of hydrophobic groups in N-isopropylacrylamide copolymer-modified silica". Anal. Chem. 69 (5): 823–830. doi:10.1021/ac961024k. PMID 9068270.
- ↑ Hideko Kanazawa; Tastuo Sunamoto; Yoshikazu Matsushima; Akihiko Kikuchi; Teruo Okano (2000). "Temperature-responsive chromatographic separation of amino acid phenylthiohydantions using aqueous media as the mobile phase". Anal. Chem. 72 (24): 5961–5966. doi:10.1021/ac0004658. PMID 11140763.
- ↑ Chikako Sakamoto; Yuji Okada; Hideko Kanazawa; Akihiko Kikuchi; Teruo Okano (2003). "Separation of catechins by temperature-responsive chromatography". Bunseki Kagaku. 52 (10): 903–906. doi: 10.2116/bunsekikagaku.52.903 .
- ↑ Hideko Kanazawa; Kazuo Yamamoto; Yoshikazu Matsushima; Nobuharu Takai; Akihiko Kikuchi; Yasuhisa Sakurai; Teruo Okano (1996). "Temperature-Responsive Chromatography Using Poly(N-isopropylacrylamide)-Modified Silica". Anal. Chem. 68 (1): 100–105. doi:10.1021/ac950359j. PMID 21619225.
- ↑ Ken Hosoya; Etsuko Sawada; Kazuhiro Kimata; Takeo Araki; Nabuo Tanaka; Jean M.J. Fréchet (1994). "In situ Surface-Selective Modification of Uniform Size Macroporous Polymer Particles with Temperature-Responsive Poly-N-isopropylacrylamide". J. Macromol. 27 (14): 3973–3976. Bibcode:1994MaMol..27.3973H. doi:10.1021/ma00092a042.
- ↑ Jun Kobayashi; Akihiko Kikuchi; Kiyotaka Sakai; Teruo Okano (2003). "Cross-linked thermoresponsive anionic polymer-grafted surfaces to separate bioactive basic peptides". Anal. Chem. 75 (13): 3244–3249. doi:10.1021/ac026364m. PMID 12964775.
- ↑ Eri Ayano; Kyoko Nambu; Chikako Sakamoto; Hideko Kanazawa; Akihiko Kikuchi; Teruo Okano (2006). "Aqueous chromatography system using pH- and temperature-responsive stationary phase with ion-exchange groups". J. Chromatogr. A. 1119 (1–2): 58–65. doi:10.1016/j.chroma.2006.01.068. PMID 16460743.
- ↑ Guohua Chen; Allan S. Hoffman (1993). "Preparation and properties of thermoreversible, phase-separating enzyme-oligo(N-isopropylacrylamide) conjugates". Bioconjugate Chem. 4 (6): 509–514. doi:10.1021/bc00024a013. PMID 8305520.
- ↑ Kazuhiro Hoshino; Masayuki Taniguchi; Taichi Kitao; Shoichi Morohashi; Toshisuke Sasakura (1998). "Preparation of a new thermo-responsive adsorbent with maltose as a ligand and its application to affinity precipitation". Biotechnology and Bioengineering. 60 (5): 568–579. doi:10.1002/(SICI)1097-0290(19981205)60:5<568::AID-BIT7>3.0.CO;2-V. PMID 10099465. S2CID 29656259.
- ↑ S. Anastase-Ravion; Z. Ding; A. Pellé; A.S. Hoffman; D. Letourneur (2001). "New antibody purification procedure using a thermally responsive poly(N-isopropylacrylamide)–dextran derivative conjugate". J. Chromatogr. B. 761 (2): 247–254. doi:10.1016/S0378-4347(01)00336-X. PMID 11587355.
- ↑ Noah Malmstadt; Paul Yager; Allan S. Hoffman; Patrick S. Stayton (2003). "A smart microfluidic affinity chromatography matrix composed of poly(N-isopropylacrylamide)-coated beads". Anal. Chem. 75 (13): 2943–2949. doi:10.1021/ac034274r. PMID 12964737.