Related Research Articles
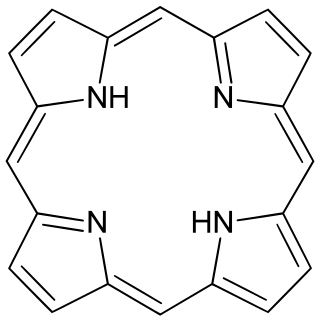
Porphyrins are a group of heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges. In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis.
The philosophy of chemistry considers the methodology and underlying assumptions of the science of chemistry. It is explored by philosophers, chemists, and philosopher-chemist teams. For much of its history, philosophy of science has been dominated by the philosophy of physics, but the philosophical questions that arise from chemistry have received increasing attention since the latter part of the 20th century.

Porphine or porphin is an organic compound of empirical formula C20H14N4. It is heterocyclic and aromatic. The molecule is a flat macrocycle, consisting of four pyrrole-like rings joined by four methine bridges, which makes it the simplest of the tetrapyrroles.
Carl Shipp "Speed" Marvel was an American chemist who specialized in polymer chemistry. He made important contributions to U.S. synthetic rubber program during World War II, and later worked at developing polybenzimidazoles, temperature-resistant polymers that are used in the aerospace industry, in fire-fighting equipment, and as a replacement for asbestos. He has been described as "one of the world's outstanding organic chemists" and received numerous awards, including the 1956 Priestley Medal and the 1986 National Medal of Science, presented by President Ronald Reagan.

Sir Alan Rushton Battersby (4 March 1925 – 10 February 2018) was an English organic chemist best known for his work to define the chemical intermediates in the biosynthetic pathway to vitamin B12 and the reaction mechanisms of the enzymes involved. His research group was also notable for its synthesis of radiolabelled precursors to study alkaloid biosynthesis and the stereochemistry of enzymic reactions. He won numerous awards including the Royal Medal in 1984 and the Copley Medal in 2000. He was knighted in the 1992 New Year Honours. Battersby died in February 2018 at the age of 92.

Irina Petrovna Beletskaya is a Soviet and Russian professor of chemistry at Moscow State University. She specializes in organometallic chemistry and its application to problems in organic chemistry. She is best known for her studies on aromatic reaction mechanisms, as well as work on carbanion acidity and reactivity. She developed some of the first methods for carbon-carbon bond formation using palladium or nickel catalysts, and extended these reactions to work in aqueous media. She also helped to open up the chemistry of organolanthanides.

Phthalonitrile is an organic compound with the formula C6H4(CN)2, which is an off-white crystal solid at room temperature. It is a derivative of benzene, containing two adjacent nitrile groups. The compound has low solubility in water but is soluble in common organic solvents. The compound is used as a precursor to phthalocyanine and other pigments, fluorescent brighteners, and photographic sensitizers.
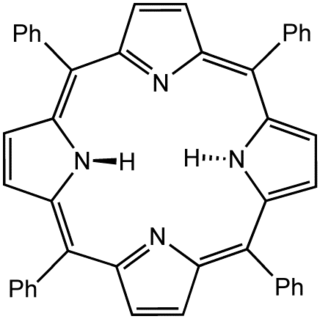
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents. Tetraphenylporphyrin is hydrophobic, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform and benzene.

Published by World Scientific, the Handbook of Porphyrin Science: With Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine is a multi-volume reference set edited by scientists Karl Kadish, Kevin Smith and Roger Guilard. The first ten volumes were published in 2010 and the next ten are expected to be published in 2011.
Jeremy Keith Morris Sanders is a British chemist and Emeritus Professor in the Department of Chemistry at the University of Cambridge. He is also Editor-in-Chief of Royal Society Open Science. He is known for his contributions to many fields including NMR spectroscopy and supramolecular chemistry. He served as the Pro-Vice-Chancellor for Institutional Affairs at the University of Cambridge, 2011–2015.
The Barton–Zard reaction is a route to pyrrole derivatives via the reaction of a nitroalkene with an α-isocyanide under basic conditions. It is named after Derek Barton and Samir Zard who first reported it in 1985.

The Journal of Porphyrins and Phthalocyanines (JPP) is a scientific journal that covers developments in the research and technology of porphyrins and phthalocyanines.
John T. Groves is an American chemist, and Hugh Stott Taylor Chair of Chemistry, at Princeton University.
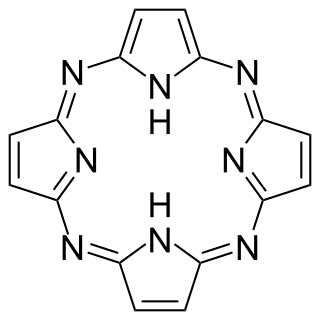
Porphyrazines, or tetraazaporphyrins, are tetrapyrrole macrocycles similar to porphyrins and phthalocyanines. Pioneered by Sir R. Patrick Linstead as an extension of his work on phthalocyanines, porphyrazines differ from porphyrins in that they contain -meso nitrogen atoms, rather than carbon atoms, and differ from phthalocyanines in that their β-pyrrole positions are open for substitution. These differences confer physical properties that are distinct from both porphyrins and phthalocyanines.

Harry Laurence Anderson is a British chemist in the Department of Chemistry, University of Oxford. He is well known for his contributions in the syntheses of supramolecular systems, exploration of the extraordinary physical properties of large pi-conjugated systems, and synthesis of cyclo[18]carbon. He is a Professor of Chemistry at Keble College, Oxford.

Karl M. Kadish is an American chemist. He is currently Hugh Roy and Lillie Cranz Cullen University Professor at the University of Houston.

Atsuhiro Osuka is a research professor of organic chemistry in the Department of Chemistry, Graduate School of Science, Kyoto University (Japan). He is recognized in the fields of porphyrinoid chemistry for his works in extended π-electron systems and its tunable aromatic behaviors.
Kay Michille Brummond is an American synthetic chemist who is Professor of Chemistry and Associate Dean of Faculty at the University of Pittsburgh. Her interests consider cycloaddition reactions that can realise molecules and natural products for organic photovoltaics and targeted covalent inhibitors. She was elected a Fellow of the American Chemical Society (ACS) in 2010, a Fellow of the AAAS in 2021, and awarded the ACS National Award for Encouraging Women into Careers in the Chemical Sciences in 2021.

Transition metal porphyrin complexes are a family of coordination complexes of the conjugate base of porphyrins. Iron porphyrin complexes occur widely in Nature, which has stimulated extensive studies on related synthetic complexes. The metal-porphyrin interaction is a strong one such that metalloporphyrins are thermally robust. They are catalysts and exhibit rich optical properties, although these complexes remain mainly of academic interest.

Phosphorus-centered porphyrins are conjugated polycyclic ring systems consisting of either four pyrroles with inward-facing nitrogens and a phosphorus atom at their core or porphyrins with one of the four pyrroles substituted for a phosphole. Unmodified porphyrins are composed of pyrroles and linked by unsaturated hydrocarbon bridges often acting as multidentate ligands centered around a transition metal like Cu II, Zn II, Co II, Fe III. Being highly conjugated molecules with many accessible energy levels, porphyrins are used in biological systems to perform light-energy conversion and modified synthetically to perform similar functions as a photoswitch or catalytic electron carriers. Phosphorus III and V ions are much smaller than the typical metal centers and bestow distinct photochemical properties unto the porphyrin. Similar compounds with other pnictogen cores or different polycyclic rings coordinated to phosphorus result in other changes to the porphyrin’s chemistry.
References
- 1 2 3 4 5 6 "Dr. Timothy Lash "Distinguished Professor, Organic Chemistry"". chemistry.illinoisstate.edu. illinoisstate.edu.
- 1 2 3 Lash, Timothy D. (November 2007). "Recent Advances on the Synthesis and Chemistry of Carbaporphyrins and Related Porphyrinoid Systems". European Journal of Organic Chemistry. 2007 (33): 5461–5481. doi:10.1002/ejoc.200700478.
- ↑ Kadish, Karl M.; Smith, Kevin M.; Guilard, Roger (2016). Handbook of Porphyrin Science – With Applications to Chemistry, Physics, Material Science, Engineering, Biology and Medicine (Volume 16) "Chapter 74 – Carbaporphyrins and Related Systems. Synthesis, Characterization, Reactivity and Insights into the Nature of Porphyrinoid Aromaticity". Vol. 40. World Scientific Publishing. doi:10.1142/10055. ISBN 978-9814417280.