Related Research Articles
Toluene, also known as toluol, is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
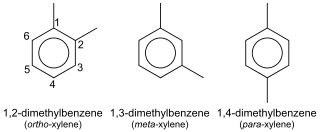
In organic chemistry, xylene or xylol are any of three organic compounds with the formula (CH3)2C6H4. They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value.

Cosmetology is the study and application of beauty treatment. Branches of specialty include hairstyling, skin care, cosmetics, manicures/pedicures, non-permanent hair removal such as waxing and sugaring, and permanent hair removal processes such as electrology and intense pulsed light (IPL).

Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics and pesticides, explosives and drugs. It has also been used to make bile acids, cholesterol and steroids.
A paint thinner is a solvent used to thin oil-based paints. Solvents labeled "paint thinner" are usually mineral spirits having a flash point at about 40 °C (104 °F), the same as some popular brands of charcoal starter.

Toluene diisocyanate (TDI) is an organic compound with the formula CH3C6H3(NCO)2. Two of the six possible isomers are commercially important: 2,4-TDI (CAS: 584-84-9) and 2,6-TDI (CAS: 91-08-7). 2,4-TDI is produced in the pure state, but TDI is often marketed as 80/20 and 65/35 mixtures of the 2,4 and 2,6 isomers respectively. It is produced on a large scale, accounting for 34.1% of the global isocyanate market in 2000, second only to MDI. Approximately 1.4 billion kilograms were produced in 2000. All isomers of TDI are colorless, although commercial samples can appear yellow.
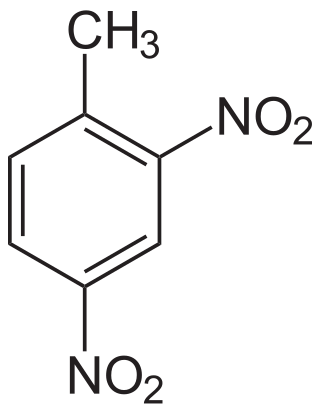
2,4-Dinitrotoluene (DNT) or dinitro is an organic compound with the formula C7H6N2O4. This pale yellow crystalline solid is well known as a precursor to trinitrotoluene (TNT) but is mainly produced as a precursor to toluene diisocyanate.
Toluene toxicity refers to the harmful effects caused by toluene on the body.

Tellurium tetrabromide (TeBr4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl4. It can be made by reacting bromine and tellurium. In the vapour TeBr4 dissociates:

Diisopropylzinc is an organozinc compound with the chemical formula ZnC6H14.
Methylcyclohexane (cyclohexylmethane) is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted in naphtha reformers to toluene. Methylcyclohexane is also used in some correction fluids (such as White-Out) as a solvent.
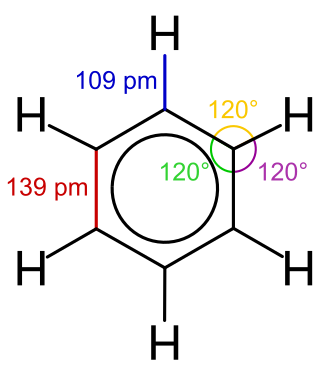
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.
Commodity chemicals are a group of chemicals that are made on a very large scale to satisfy global markets. The average prices of commodity chemicals are regularly published in the chemical trade magazines and web sites such as Chemical Week and ICIS. There have been several studies of the scale and complexity of this market for example in the USA.
1,2,3-Trimethylbenzene is an organic compound with the chemical formula C6H3(CH3)3. Classified as an aromatic hydrocarbon, it is a flammable colorless liquid. It is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar and petroleum. It is one of the three isomers of trimethylbenzene. It is used in jet fuel, mixed with other hydrocarbons, to prevent the formation of solid particles which might damage the engine.

In the petroleum refining and petrochemical industries, the initialism BTX refers to mixtures of benzene, toluene, and the three xylene isomers, all of which are aromatic hydrocarbons. The xylene isomers are distinguished by the designations ortho –, meta –, and para – as indicated in the adjacent diagram. If ethylbenzene is included, the mixture is sometimes referred to as BTEX.
Drug precursors, also referred to as precursor chemicals or simply precursors, are substances which are known to be used in the illegal manufacture of illicit drugs. Most precursors also have legitimate commercial uses and are legally used in a wide variety of industrial processes and consumer products, such as medicines, flavourings, and fragrances.
Nickel(II) titanate is an inorganic compound with the chemical formula NiTiO3 nickel(II) titanate, also known as nickel titanium oxide, is a coordination compound between nickel(II), titanium(IV) and oxide ions. It has the appearance of a yellow powder. There are several methods of synthesis for nickel(II) titanate. The first method involves nickel(II) titanate's melting temperature of over 500 °C at which its precursor decomposes to give nickel(II) titanate as a residue. Nickel(II) titanate has been used as a catalyst for toluene oxidation. The second method involved using enthalpy and entropy on the reaction to synthesize nickel(II) titanate through its phase transition.
Hazard substitution is a hazard control strategy in which a material or process is replaced with another that is less hazardous. Substitution is the second most effective of the five members of the hierarchy of hazard controls in protecting workers, after elimination. Substitution and elimination are most effective early in the design process, when they may be inexpensive and simple to implement, while for an existing process they may require major changes in equipment and procedures. The concept of prevention through design emphasizes integrating the more effective control methods such as elimination and substitution early in the design phase.

The CompTox Chemicals Dashboard is a freely accessible online database created and maintained by the U.S. Environmental Protection Agency (EPA). The database provides access to multiple types of data including physicochemical properties, environmental fate and transport, exposure, usage, in vivo toxicity, and in vitro bioassay. EPA and other scientists use the data and models contained within the dashboard to help identify chemicals that require further testing and reduce the use of animals in chemical testing. The Dashboard is also used to provide public access to information from EPA Action Plans, e.g. around perfluorinated alkylated substances.
References
- ↑ "Pure Component Database" (Queriable database). Chemical Engineering Research Information Center. Retrieved 12 May 2007.
- ↑ Lange's Handbook of Chemistry 10th ed (1967), pp 1522–1524