Methods
Application of toxic units requires toxicity data for the individual components of the mixture as well as specialized mixture toxicity data. Evaluating the response of each individual chemical allows researchers to generate a new dosing metric, toxic units, which is standardized to the toxicity of each chemical. Since the toxicity of two compounds may vary widely, 1 toxic unit of two different compounds could correspond to two very different concentrations on a per mass basis. In addition to the toxicity of the individual components, use of toxic units requires a 2x2 factorial design concentration series where the response is measured to an increase of each contaminant with the other contaminant held constant. This elaborate concentration series allows researchers to describe how the mixture components interact with each other and predict effects at untested combinations components with nonlinear regression models.
Point estimates

Point estimation is a technique to predict population parameters based on available sample data and can be used to relate the mass based concentration to a toxicity based metric. Point estimates in toxicology are frequently response endpoints on a dose response curve. These point estimates predict at what concentration one would expect to see a given biological endpoint like 50% mortality (LC50). Any toxicological endpoint (growth inhibition, reproduction, behavior etc.) can be used as the toxicity metric to convert from mass based concentration to toxic units.
Point estimates are generated by fitting a nonlinear regression model to toxicity data and using that model to predict the concentration of chemical required to elicit a known response of the biological endpoint.
Equation and calculations
One Toxic unit can be defined by the researcher as the concentration of a given chemical required to cause a given toxicological endpoint (LC50, EC50, IC50).
1TU=LC50 or 1TU=IC50 for inhibition of growth
Since the mass or molar based concentrations of different chemicals required to cause a given endpoint like an LC50 may vary widely, the concentration that corresponds to 1TU is specific to each individual chemical tested.
Isobolograms
Isobolograms are one way to present the results of binary mixture toxicity testing based in toxic units. [2] The strength of this method is its simplicity and ease of use. First a line of additivity is plotted that corresponds to all the combinations of the two chemicals that would result in one toxic unit. Next the experimental results from binary mixture tests are plotted on the isobologram. The results from the mixture test are point estimates from the mixture dose response curves that correspond to the single chemical tests. When these mixture point estimates are plotted on the isobologram, the region that they fall into (based on the concentrations of the two chemicals required to cause that given endpoint) demonstrates whether the mixture interactions are additive, synergistic or antagonistic.
Response surfaces
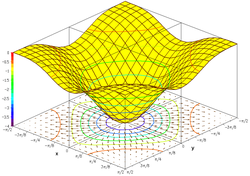
Response surfaces are a more advanced and complex way to visualize the same information presented in an isobologram. A response surface is a three dimensional graph with concentrations of individual components in toxic units on the x and y axis and the response variable on the z axis. This three dimensional representation of the organisms response to the two chemical stressors can be used to predict the toxicity of any combination of the components based on the nonlinear regression models that form the response surface. [2]
Antagonistic, additive, and synergistic effects
The primary utility of toxic units is to classify mixture interactions as additive, synergistic or antagonistic. Additivity means that the toxicity of the mixture is equal to the sum of the toxicities of the individual components. Additivity is the default assumption of models used to predict toxicity of mixtures for regulatory and environmental management purposes. Synergistic effects occur when the experimental toxicity of the mixture is greater than the sum of the individual toxicities. Conversely, antagonistic effects occur when the experimental toxicity of a mixture is less than would be predicted by additivity. Understanding mixture interactions can prevent over or underestimation of toxicity by regulators who assume additivity for uncategorized mixtures.