Related Research Articles
An allele is a variation of the same sequence of nucleotides at the same place on a long DNA molecule, as described in leading textbooks on genetics and evolution. The word is a short form of "allelomorph".
The genotype of an organism is its complete set of genetic material. Genotype can also be used to refer to the alleles or variants an individual carries in a particular gene or genetic location. The number of alleles an individual can have in a specific gene depends on the number of copies of each chromosome found in that species, also referred to as ploidy. In diploid species like humans, two full sets of chromosomes are present, meaning each individual has two alleles for any given gene. If both alleles are the same, the genotype is referred to as homozygous. If the alleles are different, the genotype is referred to as heterozygous.

Mendelian inheritance is a type of biological inheritance following the principles originally proposed by Gregor Mendel in 1865 and 1866, re-discovered in 1900 by Hugo de Vries and Carl Correns, and later popularized by William Bateson. These principles were initially controversial. When Mendel's theories were integrated with the Boveri–Sutton chromosome theory of inheritance by Thomas Hunt Morgan in 1915, they became the core of classical genetics. Ronald Fisher combined these ideas with the theory of natural selection in his 1930 book The Genetical Theory of Natural Selection, putting evolution onto a mathematical footing and forming the basis for population genetics within the modern evolutionary synthesis.

In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and the second is called recessive. This state of having two different variants of the same gene on each chromosome is originally caused by a mutation in one of the genes, either new or inherited. The terms autosomal dominant or autosomal recessive are used to describe gene variants on non-sex chromosomes (autosomes) and their associated traits, while those on sex chromosomes (allosomes) are termed X-linked dominant, X-linked recessive or Y-linked; these have an inheritance and presentation pattern that depends on the sex of both the parent and the child. Since there is only one copy of the Y chromosome, Y-linked traits cannot be dominant or recessive. Additionally, there are other forms of dominance, such as incomplete dominance, in which a gene variant has a partial effect compared to when it is present on both chromosomes, and co-dominance, in which different variants on each chromosome both show their associated traits.
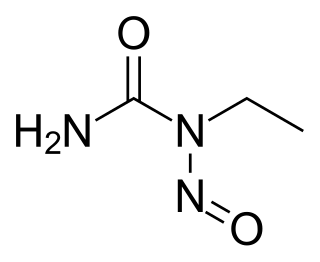
ENU, also known as N-ethyl-N-nitrosourea (chemical formula C3H7N3O2), is a highly potent mutagen. For a given gene in mice, ENU can induce 1 new mutation in every 700 loci. It is also toxic at high doses.
A heterozygote advantage describes the case in which the heterozygous genotype has a higher relative fitness than either the homozygous dominant or homozygous recessive genotype. Loci exhibiting heterozygote advantage are a small minority of loci. The specific case of heterozygote advantage due to a single locus is known as overdominance. Overdominance is a rare condition in genetics where the phenotype of the heterozygote lies outside of the phenotypical range of both homozygote parents, and heterozygous individuals have a higher fitness than homozygous individuals.

Equine coat color genetics determine a horse's coat color. Many colors are possible, but all variations are produced by changes in only a few genes. Bay is the most common color of horses. A change at the agouti locus is capable of turning bay to black, while a mutation at the extension locus can turn bay or black to chestnut.

Non-Mendelian inheritance is any pattern in which traits do not segregate in accordance with Mendel's laws. These laws describe the inheritance of traits linked to single genes on chromosomes in the nucleus. In Mendelian inheritance, each parent contributes one of two possible alleles for a trait. If the genotypes of both parents in a genetic cross are known, Mendel's laws can be used to determine the distribution of phenotypes expected for the population of offspring. There are several situations in which the proportions of phenotypes observed in the progeny do not match the predicted values.
Complementation refers to a genetic process when two strains of an organism with different homozygous recessive mutations that produce the same mutant phenotype have offspring that express the wild-type phenotype when mated or crossed. Complementation will ordinarily occur if the mutations are in different genes. Complementation may also occur if the two mutations are at different sites within the same gene, but this effect is usually weaker than that of intergenic complementation. In the case where the mutations are in different genes, each strain's genome supplies the wild-type allele to "complement" the mutated allele of the other strain's genome. Since the mutations are recessive, the offspring will display the wild-type phenotype. A complementation test can be used to test whether the mutations in two strains are in different genes. Complementation is usually weaker or absent if the mutations are in the same gene. The convenience and essence of this test is that the mutations that produce a phenotype can be assigned to different genes without the exact knowledge of what the gene product is doing on a molecular level. The complementation test was developed by American geneticist Edward B. Lewis.

Haploinsufficiency in genetics describes a model of dominant gene action in diploid organisms, in which a single copy of the wild-type allele at a locus in heterozygous combination with a variant allele is insufficient to produce the wild-type phenotype. Haploinsufficiency may arise from a de novo or inherited loss-of-function mutation in the variant allele, such that it yields little or no gene product. Although the other, standard allele still produces the standard amount of product, the total product is insufficient to produce the standard phenotype. This heterozygous genotype may result in a non- or sub-standard, deleterious, and (or) disease phenotype. Haploinsufficiency is the standard explanation for dominant deleterious alleles.
A null allele is a nonfunctional allele caused by a genetic mutation. Such mutations can cause a complete lack of production of the associated gene product or a product that does not function properly; in either case, the allele may be considered nonfunctional. A null allele cannot be distinguished from deletion of the entire locus solely from phenotypic observation.
Under the law of dominance in genetics, an individual expressing a dominant phenotype could contain either two copies of the dominant allele or one copy of each dominant and recessive allele. By performing a test cross, one can determine whether the individual is heterozygous or homozygous dominant.
Mitotic recombination is a type of genetic recombination that may occur in somatic cells during their preparation for mitosis in both sexual and asexual organisms. In asexual organisms, the study of mitotic recombination is one way to understand genetic linkage because it is the only source of recombination within an individual. Additionally, mitotic recombination can result in the expression of recessive alleles in an otherwise heterozygous individual. This expression has important implications for the study of tumorigenesis and lethal recessive alleles. Mitotic homologous recombination occurs mainly between sister chromatids subsequent to replication. Inter-sister homologous recombination is ordinarily genetically silent. During mitosis the incidence of recombination between non-sister homologous chromatids is only about 1% of that between sister chromatids.
Hermann J. Muller (1890–1967), who was a 1946 Nobel Prize winner, coined the terms amorph, hypomorph, hypermorph, antimorph and neomorph to classify mutations based on their behaviour in various genetic situations, as well as gene interaction between themselves. These classifications are still widely used in Drosophila genetics to describe mutations. For a more general description of mutations, see mutation, and for a discussion of allele interactions, see dominance relationship.
Balancer chromosomes are a type of genetically engineered chromosome used in laboratory biology for the maintenance of recessive lethal mutations within living organisms without interference from natural selection. Since such mutations are viable only in heterozygotes, they cannot be stably maintained through successive generations and therefore continually lead to production of wild-type organisms, which can be prevented by replacing the homologous wild-type chromosome with a balancer. In this capacity, balancers are crucial for genetics research on model organisms such as Drosophila melanogaster, the common fruit fly, for which stocks cannot be archived. They can also be used in forward genetics screens to specifically identify recessive lethal mutations. For that reason, balancers are also used in other model organisms, most notably the nematode worm Caenorhabditis elegans and the mouse.
In medical genetics, compound heterozygosity is the condition of having two or more heterogeneous recessive alleles at a particular locus that can cause genetic disease in a heterozygous state; that is, an organism is a compound heterozygote when it has two recessive alleles for the same gene, but with those two alleles being different from each other. Compound heterozygosity reflects the diversity of the mutation base for many autosomal recessive genetic disorders; mutations in most disease-causing genes have arisen many times. This means that many cases of disease arise in individuals who have two unrelated alleles, who technically are heterozygotes, but both the alleles are defective.
Lethal alleles are alleles that cause the death of the organism that carries them. They are usually a result of mutations in genes that are essential for growth or development. Lethal alleles may be recessive, dominant, or conditional depending on the gene or genes involved.

Zygosity is the degree to which both copies of a chromosome or gene have the same genetic sequence. In other words, it is the degree of similarity of the alleles in an organism.
Classical genetics is the branch of genetics based solely on visible results of reproductive acts. It is the oldest discipline in the field of genetics, going back to the experiments on Mendelian inheritance by Gregor Mendel who made it possible to identify the basic mechanisms of heredity. Subsequently, these mechanisms have been studied and explained at the molecular level.
This glossary of genetics and evolutionary biology is a list of definitions of terms and concepts used in the study of genetics and evolutionary biology, as well as sub-disciplines and related fields, with an emphasis on classical genetics, quantitative genetics, population biology, phylogenetics, speciation, and systematics. Overlapping and related terms can be found in Glossary of cellular and molecular biology, Glossary of ecology, and Glossary of biology.
References
- ↑ Price JV, Savenye ED, Lum D, Breitkreutz A (1997). "Dominant enhancers of Egfr in Drosophila melanogaster: genetic links between the Notch and Egfr signaling pathways". Genetics. 147 (3): 1139–53. doi:10.1093/genetics/147.3.1139. PMC 1208239 . PMID 9383058.
- ↑ Yue L, Karr TL, Nathan DF, Swift H, Srinivasan S, Lindquist S (1999). "Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis". Genetics. 151 (3): 1065–79. doi:10.1093/genetics/151.3.1065. PMC 1460532 . PMID 10049923.
- ↑ Stowers RS, Garza D, Rascle A, Hogness DS (2000). "The L63 gene is necessary for the ecdysone-induced 63E late puff and encodes CDK proteins required for Drosophila development". Dev. Biol. 221 (1): 23–40. doi: 10.1006/dbio.2000.9685 . PMID 10772789.