Related Research Articles
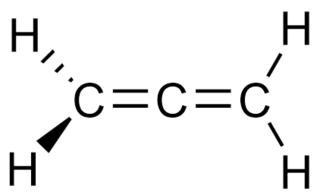
Allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding.

Azide is the anion with the formula N−
3. It is the conjugate base of hydrazoic acid (HN3). N−
3 is a linear anion that is isoelectronic with CO2, NCO−, N2O, NO+
2 and NCF. Per valence bond theory, azide can be described by several resonance structures; an important one being . Azide is also a functional group in organic chemistry, RN3.
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates.
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
Dimethylformamide is an organic compound with the formula (CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by sparging degraded samples with an inert gas such as argon or by sonicating the samples under reduced pressure. As its name indicates, it is a derivative of formamide, the amide of formic acid. DMF is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions.
Boron trifluoride is the inorganic compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
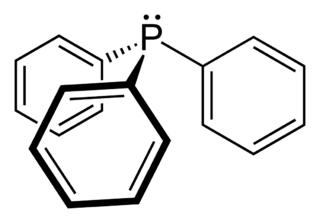
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide ((C2H5)2SnI2), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn-C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R−N+
2X−
where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halogen.

In chemistry, a polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (−C≡C−)
n with n greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds (−CR=CR′−). They are also sometimes referred to as oligoynes, or carbinoids after "carbyne" (−C≡C−)
∞, the hypothetical allotrope of carbon that would be the ultimate member of the series. The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today.
Organoselenium compounds are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

A Grignard reagent or Grignard Compound is a chemical compound with the generic formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH
3 and phenylmagnesium bromide (C
6H
5)−Mg−Br. They are a subclass of the organomagnesium compounds.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives (for example Cn(CH3)n) and heterocyclic derivatives (for example BCnHn+1). Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.

Triphenyltin hydride is the organotin compound with the formula (C6H5)3SnH. It is a white distillable oil that is soluble in organic solvents. It is often used as a source of "H·" to generate radicals or cleave carbon-oxygen bonds.
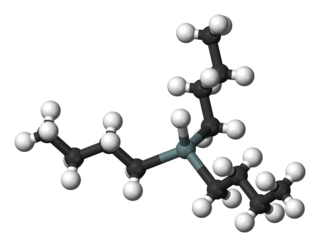
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organmetallic group 2 compounds are rare and are typically limited to academic interests.

Organozirconium compounds are organometallic compounds containing a carbon to zirconium chemical bond. Organozirconium chemistry is the corresponding science exploring properties, structure, and reactivity of these compounds. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.
References
- ↑ "Concise International Chemical Assessment Document 13: Triphenyltin Compounds". International Programme on Chemical Safety . Retrieved 2010-04-08.
- ↑ "Triphenyltin compounds". Environment Agency. Archived from the original on 2010-04-08. Retrieved 2010-04-08.
- ↑ Clive, D. L. J. (2004). "Triphenylstannane". In Paquette, L (ed.). e-EROS Encyclopedia of Reagents for Organic Synthesis . New York: J. Wiley & Sons. doi:10.1002/047084289X.rt390. ISBN 0471936235.