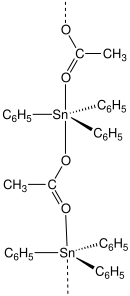
 | |
| Names | |
|---|---|
| IUPAC name (acetoxy)(triphenyl)stannane | |
| Other names Phentin acetate; Triphenyltin acetate; Triphenylstannyl acetate; Acetic acid tri(phenyl)stannyl ester, Brestan | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.804 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C20H18O2Sn | |
| Molar mass | 409.07 g/mol |
| Melting point | 122–124 °C (252–255 °F; 395–397 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Very toxic Dangerous for the environment |
| GHS labelling: | |
    | |
| Warning | |
| H301, H311, H315, H318, H330, H335, H351, H361d, H372, H410 | |
| P201, P202, P260, P264, P270, P271, P273, P280, P284, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P320, P330, P332+P313, P361, P363, P391, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 21 mg/kg (guinea pig, oral) 30 mg/kg (rabbit, oral) 81 mg/kg (mouse, oral) 125 mg/kg (rat, oral) [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Fentin acetate is an organotin compound with the formula (C6H5)3SnO2CCH3. It is a colourless solid that was previously used as a fungicide. [3] [4]