
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."

Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states. For this reason salen-type compounds are used as metal deactivators. Metal salen complexes also find use as catalysts.
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular H2. It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular H2 used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to CO2, acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin.
In chemistry, bis(oxazoline) ligands (often abbreviated BOX ligands) are a class of privileged chiral ligands containing two oxazoline rings. They are typically C2‑symmetric and exist in a wide variety of forms; with structures based around CH2 or pyridine linkers being particularly common (often generalised BOX and PyBOX respectively). The coordination complexes of bis(oxazoline) ligands are used in asymmetric catalysis. These ligands are examples of C2-symmetric ligands.

DIPAMP is an organophosphorus compound that is used as a ligand in homogeneous catalysis. It is a white solid that dissolves in organic solvents. Work on this compound by W. S. Knowles was recognized with the Nobel Prize in Chemistry. DIPAMP was the basis for of the first industrial scale asymmetric hydrogenation, the synthesis of the drug L-DOPA.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate many novel synthetic molecules that interact with biological systems in specific ways, leading to new pharmaceutical agents and agrochemicals. The importance of asymmetric hydrogenation in both academia and industry contributed to two of its pioneers — William Standish Knowles and Ryōji Noyori — being awarded one half of the 2001 Nobel Prize in Chemistry.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.

Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.
Cyclodiphosphazanes are saturated four membered P2N2 ring systems and one of the major classes of cyclic phosphazene compounds. Bis(chloro)cyclodiphosphazanes, (cis-[ClP(μ-NR)]2) are important starting compounds for synthesizing a variety of cyclodiphosphazane derivatives by nucleophilic substitution reactions; are prepared by reaction of phosphorus trichloride (PCl3) with a primary amine (RNH2) or amine hydrochlorides (RNH3Cl).

DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding. The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective applications.
The Tsuji–Trost reaction is a palladium-catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst first coordinates with the allyl group and then undergoes oxidative addition, forming the π-allyl complex. This allyl complex can then be attacked by a nucleophile, resulting in the substituted product.

A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.
In chemistry, metal-catalysed hydroboration is a reaction used in organic synthesis. It is one of several examples of homogeneous catalysis.
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early and late d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen, nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.

Phosphinooxazolines are a class of chiral ligands used in asymmetric catalysis. Their complexes are particularly effective at generating single enatiomers in reactions involving highly symmetric transition states, such as allylic substitutions, which are typically difficult to perform stereoselectively. The ligands are bidentate and have been shown to be hemilabile with the softer P‑donor being more firmly bound than the harder N‑donor.
Andreas Pfaltz is a Swiss chemist known for his work in the area of coordination chemistry and catalysis.
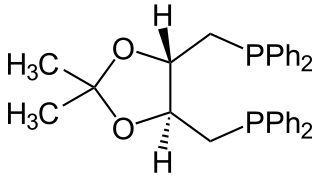
DIOP is an organophosphorus compound that is used as a chiral ligand in asymmetric catalysis. It is a white solid that is soluble in organic solvents.
In homogeneous catalysis, C2-symmetric ligands refer to ligands that lack mirror symmetry but have C2 symmetry. Such ligands are usually bidentate and are valuable in catalysis. The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. C2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including C2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.













