Related Research Articles

In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30-nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 90 micrometers (0.09 um) of 30 nm diameter chromatin fibers.

A retrovirus is a type of virus that inserts a copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. Once inside the host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus.

A transposable element is a DNA sequence that can change its position within a genome, sometimes creating or reversing mutations and altering the cell's genetic identity and genome size. Transposition often results in duplication of the same genetic material. Barbara McClintock's discovery of them earned her a Nobel Prize in 1983.
Heterochromatin is a tightly packed form of DNA or condensed DNA, which comes in multiple varieties. These varieties lie on a continuum between the two extremes of constitutive heterochromatin and facultative heterochromatin. Both play a role in the expression of genes. Because it is tightly packed, it was thought to be inaccessible to polymerases and therefore not transcribed; however, according to Volpe et al. (2002), and many other papers since, much of this DNA is in fact transcribed, but it is continuously turned over via RNA-induced transcriptional silencing (RITS). Recent studies with electron microscopy and OsO4 staining reveal that the dense packing is not due to the chromatin.

Retrotransposons are a type of genetic component that copy and paste themselves into different genomic locations (transposon) by converting RNA back into DNA through the process reverse transcription using an RNA transposition intermediate.
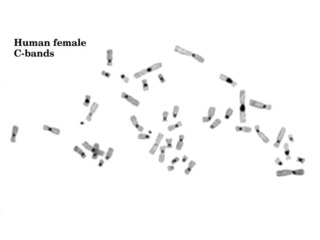
Constitutive heterochromatin domains are regions of DNA found throughout the chromosomes of eukaryotes. The majority of constitutive heterochromatin is found at the pericentromeric regions of chromosomes, but is also found at the telomeres and throughout the chromosomes. In humans there is significantly more constitutive heterochromatin found on chromosomes 1, 9, 16, 19 and Y. Constitutive heterochromatin is composed mainly of high copy number tandem repeats known as satellite repeats, minisatellite and microsatellite repeats, and transposon repeats. In humans these regions account for about 200Mb or 6.5% of the total human genome, but their repeat composition makes them difficult to sequence, so only small regions have been sequenced.

Endogenous retroviruses (ERVs) are endogenous viral elements in the genome that closely resemble and can be derived from retroviruses. They are abundant in the genomes of jawed vertebrates, and they comprise up to 5–8% of the human genome.
Subtelomeres are segments of DNA between telomeric caps and chromatin.
An insulator is a type of cis-regulatory element known as a long-range regulatory element. Found in multicellular eukaryotes and working over distances from the promoter element of the target gene, an insulator is typically 300 bp to 2000 bp in length. Insulators contain clustered binding sites for sequence specific DNA-binding proteins and mediate intra- and inter-chromosomal interactions.
Exon shuffling is a molecular mechanism for the formation of new genes. It is a process through which two or more exons from different genes can be brought together ectopically, or the same exon can be duplicated, to create a new exon-intron structure. There are different mechanisms through which exon shuffling occurs: transposon mediated exon shuffling, crossover during sexual recombination of parental genomes and illegitimate recombination.

A long terminal repeat (LTR) is a pair of identical sequences of DNA, several hundred base pairs long, which occur in eukaryotic genomes on either end of a series of genes or pseudogenes that form a retrotransposon or an endogenous retrovirus or a retroviral provirus. All retroviral genomes are flanked by LTRs, while there are some retrotransposons without LTRs. Typically, an element flanked by a pair of LTRs will encode a reverse transcriptase and an integrase, allowing the element to be copied and inserted at a different location of the genome. Copies of such an LTR-flanked element can often be found hundreds or thousands of times in a genome. LTR retrotransposons comprise about 8% of the human genome.
The family of heterochromatin protein 1 (HP1) consists of highly conserved proteins, which have important functions in the cell nucleus. These functions include gene repression by heterochromatin formation, transcriptional activation, regulation of binding of cohesion complexes to centromeres, sequestration of genes to the nuclear periphery, transcriptional arrest, maintenance of heterochromatin integrity, gene repression at the single nucleosome level, gene repression by heterochromatization of euchromatin, and DNA repair. HP1 proteins are fundamental units of heterochromatin packaging that are enriched at the centromeres and telomeres of nearly all eukaryotic chromosomes with the notable exception of budding yeast, in which a yeast-specific silencing complex of SIR proteins serve a similar function. Members of the HP1 family are characterized by an N-terminal chromodomain and a C-terminal chromoshadow domain, separated by a hinge region. HP1 is also found at some euchromatic sites, where its binding can correlate with either gene repression or gene activation. HP1 was originally discovered by Tharappel C James and Sarah Elgin in 1986 as a factor in the phenomenon known as position effect variegation in Drosophila melanogaster.

AIDS is caused by the human immunodeficiency virus (HIV). Individuals with HIV have what is referred to as a "HIV infection". When infected semen, vaginal secretions, or blood come in contact with the mucous membranes or broken skin of an uninfected person, HIV may be transferred to the uninfected person, causing another infection. Additionally, HIV can also be passed from infected pregnant women to their uninfected baby during pregnancy and/or delivery, or via breastfeeding. As a result of HIV infection, a portion of these individuals will progress and go on to develop clinically significant AIDS.

LTR retrotransposons are class I transposable element characterized by the presence of long terminal repeats (LTRs) directly flanking an internal coding region. As retrotransposons, they mobilize through reverse transcription of their mRNA and integration of the newly created cDNA into another location. Their mechanism of retrotransposition is shared with retroviruses, with the difference that most LTR-retrotransposons do not form infectious particles that leave the cells and therefore only replicate inside their genome of origin. Those that do (occasionally) form virus-like particles are classified under Ortervirales.
Cryptic unstable transcripts (CUTs) are a subset of non-coding RNAs (ncRNAs) that are produced from intergenic and intragenic regions. CUTs were first observed in S. cerevisiae yeast models and are found in most eukaryotes. Some basic characteristics of CUTs include a length of around 200–800 base pairs, a 5' cap, poly-adenylated tail, and rapid degradation due to the combined activity of poly-adenylating polymerases and exosome complexes. CUT transcription occurs through RNA Polymerase II and initiates from nucleosome-depleted regions, often in an antisense orientation. To date, CUTs have a relatively uncharacterized function but have been implicated in a number of putative gene regulation and silencing pathways. Thousands of loci leading to the generation of CUTs have been described in the yeast genome. Additionally, stable uncharacterized transcripts, or SUTs, have also been detected in cells and bear many similarities to CUTs but are not degraded through the same pathways.

Transcription activator-like effector nucleases (TALEN) are restriction enzymes that can be engineered to cut specific sequences of DNA. They are made by fusing a TAL effector DNA-binding domain to a DNA cleavage domain. Transcription activator-like effectors (TALEs) can be engineered to bind to practically any desired DNA sequence, so when combined with a nuclease, DNA can be cut at specific locations. The restriction enzymes can be introduced into cells, for use in gene editing or for genome editing in situ, a technique known as genome editing with engineered nucleases. Alongside zinc finger nucleases and CRISPR/Cas9, TALEN is a prominent tool in the field of genome editing.
SilentInformationRegulator (SIR) proteins are involved in regulating gene expression. SIR proteins organize heterochromatin near telomeres, rDNA, and at silent loci including hidden mating type loci in yeast. The SIR family of genes encodes catalytic and non-catalytic proteins that are involved in de-acetylation of histone tails and the subsequent condensation of chromatin around a SIR protein scaffold. Some SIR family members are conserved from yeast to humans.

Daniel Voytas, Ph.D., is Professor of Genetics, Cell Biology and Development at the University of Minnesota and Director of the Center for Precision Plant Genomics. He is also the Chief Scientific Officer of Calyxt, an agricultural biotechnology company focused on developing crops that provide consumer benefit.

Thomas Jenuwein is a German scientist working in the fields of epigenetics, chromatin biology, gene regulation and genome function.

RNA-directed DNA methylation (RdDM) is a biological process in which non-coding RNA molecules direct the addition of DNA methylation to specific DNA sequences. The RdDM pathway is unique to plants, although other mechanisms of RNA-directed chromatin modification have also been described in fungi and animals. To date, the RdDM pathway is best characterized within angiosperms, and particularly within the model plant Arabidopsis thaliana. However, conserved RdDM pathway components and associated small RNAs (sRNAs) have also been found in other groups of plants, such as gymnosperms and ferns. The RdDM pathway closely resembles other sRNA pathways, particularly the highly conserved RNAi pathway found in fungi, plants, and animals. Both the RdDM and RNAi pathways produce sRNAs and involve conserved Argonaute, Dicer and RNA-dependent RNA polymerase proteins.
References
- ↑ Kim JM, Vanguri S, Boeke JD, Gabriel A, Voytas DF (May 1998). "Transposable elements and genome organization: a comprehensive survey of retrotransposons revealed by the complete Saccharomyces cerevisiae genome sequence". Genome Res. 8 (5): 464–78. doi: 10.1101/gr.8.5.464 . PMID 9582191.
- ↑ Lesage P, Todeschini AL (2005). "Happy together: the life and times of Ty retrotransposons and their hosts". Cytogenet. Genome Res. 110 (1–4): 70–90. doi:10.1159/000084940. PMID 16093660. S2CID 40913336.
- ↑ Sandmeyer SB, Hansen LJ, Chalker DL (1990). "Integration specificity of retrotransposons and retroviruses". Annu. Rev. Genet. 24: 491–518. doi:10.1146/annurev.ge.24.120190.002423. PMID 1965102.
- 1 2 Gai X, Voytas DF (June 1998). "A single amino acid change in the yeast retrotransposon Ty5 abolishes targeting to silent chromatin". Mol. Cell. 1 (7): 1051–5. doi: 10.1016/S1097-2765(00)80105-7 . PMID 9651588.
- ↑ Zagulski M, Herbert CJ, Rytka J (1998). "Sequencing and functional analysis of the yeast genome". Acta Biochim. Pol. 45 (3): 627–43. doi: 10.18388/abp.1998_4201 . PMID 9918489.
- ↑ Zou S, Wright DA, Voytas DF (January 1995). "The Saccharomyces Ty5 retrotransposon family is associated with origins of DNA replication at the telomeres and the silent mating locus HMR". Proc. Natl. Acad. Sci. U.S.A. 92 (3): 920–4. Bibcode:1995PNAS...92..920Z. doi: 10.1073/pnas.92.3.920 . PMC 42732 . PMID 7846079.
- ↑ Zou S, Kim JM, Voytas DF (December 1996). "The Saccharomyces retrotransposon Ty5 influences the organization of chromosome ends". Nucleic Acids Res. 24 (23): 4825–31. doi:10.1093/nar/24.23.4825. PMC 146320 . PMID 8972872.
- ↑ Rine J, Herskowitz I (May 1987). "Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae". Genetics. 116 (1): 9–22. doi:10.1093/genetics/116.1.9. PMC 1203125 . PMID 3297920.
- ↑ Tanner KG, Landry J, Sternglanz R, Denu JM (December 2000). "Silent information regulator 2 family of NAD- dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose". Proc. Natl. Acad. Sci. U.S.A. 97 (26): 14178–82. Bibcode:2000PNAS...9714178T. doi: 10.1073/pnas.250422697 . PMC 18891 . PMID 11106374.
- ↑ Pryde FE, Louis EJ (November 1997). "Saccharomyces cerevisiae telomeres. A review". Biochemistry Mosc. 62 (11): 1232–41. PMID 9467847.