Related Research Articles

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements.
Durotaxis is a form of cell migration in which cells are guided by rigidity gradients, which arise from differential structural properties of the extracellular matrix (ECM). Most normal cells migrate up rigidity gradients.
Protease-activated receptors(PAR) are a subfamily of related G protein-coupled receptors that are activated by cleavage of part of their extracellular domain. They are highly expressed in platelets, and also on endothelial cells, fibroblasts, immune cells, myocytes, neurons, and tissues that line the gastrointestinal tract.
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic development, wound healing and immune responses all require the orchestrated movement of cells in particular directions to specific locations. Cells often migrate in response to specific external signals, including chemical signals and mechanical signals. Errors during this process have serious consequences, including intellectual disability, vascular disease, tumor formation and metastasis. An understanding of the mechanism by which cells migrate may lead to the development of novel therapeutic strategies for controlling, for example, invasive tumour cells.

In cell biology, focal adhesions are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects of a cell in response to ECM adhesion.

Podosomes are conical, actin-rich structures found on the outer surface of the plasma membrane of animal cells. Their size ranges from approximately 0.5 µm to 2.0 µm in diameter. While usually situated on the periphery of the cellular membrane, these unique structures display a polarized pattern of distribution in migrating cells, situating at the front border between the lamellipodium and lamellum. Their primary purpose is connected to cellular motility and invasion; therefore, they serve as both sites of attachment and degradation along the extracellular matrix. Many different specialized cells exhibit these dynamic structures such as invasive cancer cells, osteoclasts, vascular smooth muscle cells, endothelial cells, and certain immune cells like macrophages and dendritic cells.
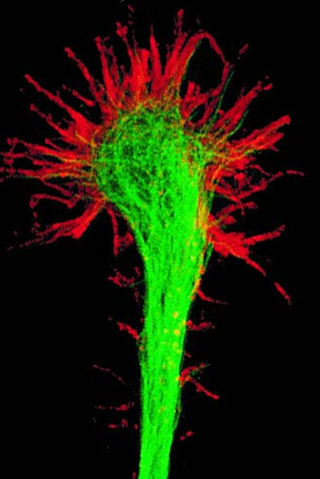
A growth cone is a large actin-supported extension of a developing or regenerating neurite seeking its synaptic target. It is the growth cone that drives axon growth. Their existence was originally proposed by Spanish histologist Santiago Ramón y Cajal based upon stationary images he observed under the microscope. He first described the growth cone based on fixed cells as "a concentration of protoplasm of conical form, endowed with amoeboid movements". Growth cones are situated on the tips of neurites, either dendrites or axons, of the nerve cell. The sensory, motor, integrative, and adaptive functions of growing axons and dendrites are all contained within this specialized structure.

ICAM-1 also known as CD54 is a protein that in humans is encoded by the ICAM1 gene. This gene encodes a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. It binds to integrins of type CD11a / CD18, or CD11b / CD18 and is also exploited by rhinovirus as a receptor for entry into respiratory epithelium.

In immunology, an immunological synapse is the interface between an antigen-presenting cell or target cell and a lymphocyte such as a T/B cell or Natural Killer cell. The interface was originally named after the neuronal synapse, with which it shares the main structural pattern. An immunological synapse consists of molecules involved in T cell activation, which compose typical patterns—activation clusters. Immunological synapses are the subject of much ongoing research.
Lymphocyte function-associated antigen 1 (LFA-1) is an integrin found on lymphocytes and other leukocytes. LFA-1 plays a key role in emigration, which is the process by which leukocytes leave the bloodstream to enter the tissues. LFA-1 also mediates firm arrest of leukocytes. Additionally, LFA-1 is involved in the process of cytotoxic T cell mediated killing as well as antibody mediated killing by granulocytes and monocytes. As of 2007, LFA-1 has 6 known ligands: ICAM-1, ICAM-2, ICAM-3, ICAM-4, ICAM-5, and JAM-A. LFA-1/ICAM-1 interactions have recently been shown to stimulate signaling pathways that influence T cell differentiation. LFA-1 belongs to the integrin superfamily of adhesion molecules.

In molecular biology, CD18 is an integrin beta chain protein that is encoded by the ITGB2 gene in humans. Upon binding with one of a number of alpha chains, CD18 is capable of forming multiple heterodimers, which play significant roles in cellular adhesion and cell surface signaling, as well as important roles in immune responses. CD18 also exists in soluble, ligand binding forms. Deficiencies in CD18 expression can lead to adhesion defects in circulating white blood cells in humans, reducing the immune system's ability to fight off foreign invaders.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.

Ezrin also known as cytovillin or villin-2 is a protein that in humans is encoded by the EZR gene.

Intercellular adhesion molecule 3 (ICAM3) also known as CD50, is a protein that in humans is encoded by the ICAM3 gene. The protein is constitutively expressed on the surface of leukocytes, which are also called white blood cells and are part of the immune system. ICAM3 mediates adhesion between cells by binding to specific integrin receptors. It plays an important role in the immune cell response through its facilitation of interactions between T cells and dendritic cells, which allows for T cell activation. ICAM3 also mediates the clearance of cells undergoing apoptosis by attracting and binding macrophages, a type of cell that breaks down infected or dying cells through a process known as phagocytosis, to apoptotic cells.

Intercellular adhesion molecule 2 (ICAM2), also known as CD102, is a human gene, and the protein resulting from it.

Moesin is a protein that in humans is encoded by the MSN gene.
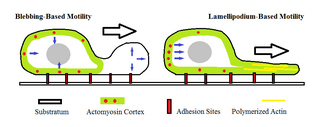
Amoeboid movement is the most typical mode of locomotion in adherent eukaryotic cells. It is a crawling-like type of movement accomplished by protrusion of cytoplasm of the cell involving the formation of pseudopodia ("false-feet") and posterior uropods. One or more pseudopodia may be produced at a time depending on the organism, but all amoeboid movement is characterized by the movement of organisms with an amorphous form that possess no set motility structures.
Cell–cell interaction refers to the direct interactions between cell surfaces that play a crucial role in the development and function of multicellular organisms. These interactions allow cells to communicate with each other in response to changes in their microenvironment. This ability to send and receive signals is essential for the survival of the cell. Interactions between cells can be stable such as those made through cell junctions. These junctions are involved in the communication and organization of cells within a particular tissue. Others are transient or temporary such as those between cells of the immune system or the interactions involved in tissue inflammation. These types of intercellular interactions are distinguished from other types such as those between cells and the extracellular matrix. The loss of communication between cells can result in uncontrollable cell growth and cancer.
For cancer, invasion is the direct extension and penetration by cancer cells into neighboring tissues. It is generally distinguished from metastasis, which is the spread of cancer cells through the circulatory system or the lymphatic system to more distant locations. Yet, lymphovascular invasion is generally the first step of metastasis.
References
- 1 2 3 4 5 6 7 8 Vicente-Manzanares, Miguel; Sánchez-Madrid, Francisco (2004). "Role of the cytoskeleton during leukocyte responses". Nature Immunology. 4 (2): 110–122. doi: 10.1038/nri1268 . PMID 15040584. S2CID 24315156.
- 1 2 3 4 5 6 7 8 9 Friedl, Peter; Weigelin, Bettina (2008). "Interstitial leukocyte migration and immune function". Nature Immunology. 9 (9): 960–969. doi:10.1038/ni.f.212. PMID 18711433. S2CID 28470211.
- 1 2 3 Burkhardt, Janis (2013). "Cytoskeletal function in the immune system". Immunological Reviews. 256 (1): 5–9. doi: 10.1111/imr.12121 . PMID 24117809.
- 1 2 3 4 Hind, Laurel; Vincent, William; Huttenlocher, Anna (2016). "Leading from the Back: The Role of the Uropod in Neutrophil Polarization and Migration". Developmental Cell. 38 (2): 161–169. doi:10.1016/j.devcel.2016.06.031. PMC 4982870 . PMID 27459068.
- 1 2 3 4 5 6 7 8 9 10 11 Sánchez-Madrid, Francisco; Serrador, Juan M. (2009). "Bringing up the rear: defining the roles of the uropod". Nature Reviews Molecular Cell Biology. 10 (5): 353–359. doi:10.1038/nrm2680. PMID 19373240. S2CID 23040367.
- 1 2 3 Nourshargh, Sussan; Alon, Ronen (2014). "Leukocyte Migration into Inflamed Tissues". Immunity Review. 41 (5): 694–707. doi: 10.1016/j.immuni.2014.10.008 . PMID 25517612.